Abstract
Background
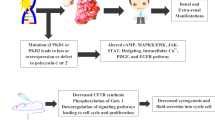
Autosomal dominant polycystic kidney disease (ADPKD) is a monogenic kidney disorder that impairs renal functions progressively leading to kidney failure. The disease affects between 1:400 and 1:1000 ratio of the people worldwide. It is caused by the mutated PKD1 and PKD2 genes which encode for the defective polycystins. Polycystins mimic the receptor protein or protein channel and mediate aberrant cell signaling that causes cystic development in the renal parenchyma. The cystic development is driven by the increased cyclic AMP stimulating fluid secretion and infinite cell growth. In recent years, natural product-derived small molecules or drugs targeting specific signaling pathways have caught attention in the drug discovery discipline. The advantages of natural products over synthetic drugs enthusiast researchers to utilize the medicinal benefits in various diseases including ADPKD.
Conclusion
Overall, this review discusses some of the previously studied and reported natural products and their mechanisms of action which may potentially be redirected into ADPKD.

Similar content being viewed by others
References
Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1(1):148–57.
Chebib FT, Torres VE. Autosomal dominant polycystic kidney disease: core curriculum 2016. Am J Kidney Dis. 2016;67(5):792–810.
Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol. 2014;25(1):18–32.
Mustafa RA, Alan SJ. Burden of proof for tolvaptan in ADPKD: did REPRISE provide the answer? Clin J Am Soc Nephrol. 2018;13(7):1107–9.
Blair HA, Keating GM. Tolvaptan: a review in autosomal dominant polycystic kidney disease. Drugs. 2015;75(15):1797–806.
Calixto JB. The role of natural products in modern drug discovery. An Acad Bras Cienc. 2019;91 Suppl 3:e20190105.
Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47.
Liu Y, Luo X, Yang C, Yang T, Zhou J, Shi S. Impact of quercetin-induced changes in drug-metabolizing enzyme and transporter expression on the pharmacokinetics of cyclosporine in rats. Mol Med Rep. 2016;14(4):3073–85.
Persu A, Devuyst O. Transepithelial chloride secretion and cystogenesis in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2000;15(6):747–50.
Graffe CC, Bech JN, Lauridsen TG, Pedersen EB. Urinary excretion of AQP2 and ENaC in autosomal dominant polycystic kidney disease during basal conditions and after a hypertonic saline infusion. Am J Physiol Renal Physiol. 2012;302(8):F917–27.
Zaika O, Mamenko M, Staruschenko A, Pochynyuk O. Direct activation of ENaC by angiotensin II: recent advances and new insights. Curr Hypertens Rep. 2013;15(1):17–24.
Zhu Y, Teng T, Wang H, Guo H, Du L, Yang B, et al. Quercetin inhibits renal cyst growth in vitro and via parenteral injection in a polycystic kidney disease mouse model. Food Funct. 2018;9(1):389–96.
Harris Z, Donovan MG, Branco GM, Limesand KH, Burd R. Quercetin as an emerging anti-melanoma agent: a four-focus area therapeutic development strategy. Front Nutr. 2016;3:48.
Rafiq RA, Quadri A, Nazir LA, Peerzada K, Ganai BA, Tasduq SA. A potent inhibitor of phosphoinositide 3-kinase (PI3K) and mitogen activated protein (MAP) kinase signalling, quercetin (3,3(,4(,5,7-pentahydroxyflavone) promotes cell death in ultraviolet (UV)-B-irradiated B16F10 melanoma cells. PLoS ONE. 2015;10(7):e0131253.
Waheed A, Ludtmann M, Pakes N, Robery S, Kuspa A, Dinh C, et al. Naringenin inhibits the growth of D ictyostelium and MDCK-derived cysts in a TRPP2 (polycystin-2)-dependent manner. Br J Pharmacol. 2014;171(10):2659–70.
Yang Y, Ehrlich BE. Structural studies of the C-terminal tail of polycystin-2 (PC2) reveal insights into the mechanisms used for the functional regulation of PC2. J Physiol. 2016;594(15):4141–9.
Fragiadaki M, Lannoy M, Themanns M, Maurer B, Leonhard WN, Peters DJ, et al. STAT5 drives abnormal proliferation in autosomal dominant polycystic kidney disease. Kidney Int. 2017;91(3):575–86.
Song HM, Park GH, Eo HJ, Lee JW, Kim MK, Lee JR, et al. Anti-proliferative effect of naringenin through p38-dependent downregulation of cyclin D1 in human colorectal cancer cells. Biomol Ther (Seoul). 2015;23(4):339.
Wang Z, Wang S, Zhao J, Yu C, Hu Y, Tu Y, et al. Naringenin ameliorates renovascular hypertensive renal damage by normalizing the balance of renin–angiotensin system components in rats. Int J Med Sci. 2019;16(5):644.
Bernstein KE, Khan Z, Giani JF, Cao D-Y, Bernstein EA, Shen XZ. Angiotensin-converting enzyme in innate and adaptive immunity. Nat Rev Nephrol. 2018;14(5):325.
Loghman-Adham M, Soto CE, Inagami T, Cassis L. The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol. 2004;287(4):F775–88.
Rinschen MM, Schermer B, Benzing T. Vasopressin-2 receptor signaling and autosomal dominant polycystic kidney disease: from bench to bedside and back again. J Am Soc Nephrol. 2014;25(6):1140–7.
Hama T, Park F. Heterotrimeric G protein signaling in polycystic kidney disease. Physiol Genomics. 2016;48(7):429–45.
Gonçalves LM, Valente IM, Rodrigues JA. An overview on cardamonin. J Med Food. 2014;17(6):633–40.
He J, Zhou H, Meng J, Zhang S, Li X, Wang S, et al. Cardamonin retards progression of autosomal dominant polycystic kidney disease via inhibiting renal cyst growth and interstitial fibrosis. Pharmacol Res. 2020;155:104751.
Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213–7.
Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res. 2009;19(1):128–39.
Norman J. Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD). Biochim Biophys Acta. 2011;1812(10):1327–36.
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong J, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98.
Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(3):607–14.
Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62.
Johanns M, Lai Y-C, Hsu M-F, Jacobs R, Vertommen D, Van Sande J, et al. AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B. Nat Commun. 2016;7(1):1–12.
Cheng SH, Rich DP, Marshall J, Gregory RJ, Welsh MJ, Smith AE. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991;66(5):1027–36.
Salani B, Del Rio A, Marini C, Sambuceti G, Cordera R, Maggi D. Metformin, cancer and glucose metabolism. Endocr Relat Cancer. 2014;21(6):R461–71.
Wang X, Ren Y. Rheum tanguticum, an endangered medicinal plant endemic to China. J Med Plants Res. 2009;3(13):1195–203.
Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Zhongguo Yao Li Xue Bao Acta Pharmacologica Sinica. 1996;86(1):147–57.
Li F, Wang S-C, Wang X, Ren Q-Y, Wang W, Shang G-W, et al. Novel exploration of cathartic pharmacology induced by rhubarb. Zhongguo Zhong Yao Za Zhi China J Chin Mater Med. 2008;33(4):481.
Zhou X, Chen Q. Biochemical study of Chinese rhubarb. XXII. Inhibitory effect of anthraquinone derivatives on Na+-K+-ATPase of the rabbit renal medulla and their diuretic action. Yao Xue Xue Bao Acta pharmaceutica Sinica. 1988;23(1):17.
Asawa RR, Danchik C, Zahkarov A, Chen Y, Voss T, Jadhav A, et al. A high-throughput screening platform for polycystic kidney disease (PKD) drug repurposing utilizing murine and human ADPKD cells. Sci Rep. 2020;10(1):1–12.
Dong X, Fu J, Yin X, Cao S, Li X, Lin L, et al. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother Res. 2016;30(8):1207–18.
Jaiswal AS, Marlow BP, Gupta N, Narayan S. β-catenin-mediated transactivation and cell–cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21(55):8414–27.
Sun Y, Wang X, Zhou Q, Lu Y, Zhang H, Chen Q, et al. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol Rep. 2015;33(1):338–46.
Zhang X, Chen Y, Zhang T, Zhang Y. Inhibitory effect of emodin on human hepatoma cell line SMMC-7721 and its mechanism. Afr Health Sci. 2015;15(1):97–100.
Xue J, Ding W, Liu Y. Anti-diabetic effects of emodin involved in the activation of PPARγ on high-fat diet-fed and low dose of streptozotocin-induced diabetic mice. Fitoterapia. 2010;81(3):173–7.
Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–23.
Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19(12):20091–112.
Patera F, Cudzich-Madry A, Huang Z, Fragiadaki M. Renal expression of JAK2 is high in polycystic kidney disease and its inhibition reduces cystogenesis. Sci Rep. 2019;9(1):1–10.
Hahn Y-I, Kim S-J, Choi B-Y, Cho K-C, Bandu R, Kim KP, et al. Curcumin interacts directly with the Cysteine 259 residue of STAT3 and induces apoptosis in H-Ras transformed human mammary epithelial cells. Sci Rep. 2018;8(1):1–14.
Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280(20):20059–68.
Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor–κB and IκBα kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101(3):1053–62.
Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21(57):8852–61.
Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, et al. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol. 2011;300(5):F1193–202.
Borges GA, Elias ST, Amorim B, de Lima CL, Coletta RD, Castilho RM, et al. Curcumin downregulates the PI3K–AKT–mTOR pathway and inhibits growth and progression in head and neck cancer cells. Phytother Res. 2020;34:3311–24.
Cianciulli A, Calvello R, Porro C, Trotta T, Salvatore R, Panaro MA. PI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int Immunopharmacol. 2016;36:282–90.
Woo J-H, Kim Y-H, Choi Y-J, Kim D-G, Lee K-S, Bae JH, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-X L and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24(7):1199–208.
Hanson J, De Oliveira B. Stevioside and related sweet diterpenoid glycosides. Nat Prod Rep. 1993;10(3):301–9.
Shibata H, Sawa Y, Oka T-A, Sonoke S, Kim KK, Yoshioka M, et al. Steviol and steviol-glycoside: glucosyltransferase activities in Stevia rebaudiana Bertoni-purification and partial characterization. Arch Biochem Biophys. 1995;321(2):390–6.
Chatsudthipong V, Muanprasat CJP, therapeutics. Stevioside and related compounds: therapeutic benefits beyond sweetness. Pharmacol Ther. 2009;121(1):41–54.
Yuajit C, Muanprasat C, Gallagher A-R, Fedeles SV, Kittayaruksakul S, Homvisasevongsa S, et al. Steviol retards renal cyst growth through reduction of CFTR expression and inhibition of epithelial cell proliferation in a mouse model of polycystic kidney disease. Biochem Pharmacol. 2014;88(3):412–21.
Xu J, Ji J, Yan X-H. Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr. 2012;52(5):373–81.
Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(20):3589–94.
Noitem R, Yuajit C, Soodvilai S, Muanprasat C, Chatsudthipong V. Steviol slows renal cyst growth by reducing AQP2 expression and promoting AQP2 degradation. Biomed Pharmacother. 2018;101:754–62.
Leuenroth SJ, Crews CM. Studies on calcium dependence reveal multiple modes of action for triptolide. Chem Biol. 2005;12(12):1259–68.
Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci. 2007;104(11):4389–94.
Leuenroth SJ, Bencivenga N, Igarashi P, Somlo S, Crews CM. Triptolide reduces cystogenesis in a model of ADPKD. J Am Soc Nephrol. 2008;19(9):1659–62.
Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu P-N, et al. PKD1 induces p21waf1 and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109(2):157–68.
Jing Y, Wu M, Zhang D, Chen D, Yang M, Mei S, et al. Triptolide delays disease progression in an adult rat model of polycystic kidney disease through the JAK2–STAT3 pathway. Am J Physiol Renal Physiol. 2018;315(3):F479–86.
Bertelli AA, Das DK. Grapes, wines, resveratrol, and heart health. J Cardiovasc Pharmacol. 2009;54(6):468–76.
Saldanha JF, Leal VDO, Stenvinkel P, Carraro-Eduardo JC, Mafra D. Resveratrol: why is it a promising therapy for chronic kidney disease patients? Oxid Med Cell Longev. 2013;2013:963217.
Kitada M, Koya D. Renal protective effects of resveratrol. Oxid Med Cell Longev. 2013;2013:568093.
Moradi H, Vaziri ND. Effect of resveratrol on progression of polycystic kidney disease: a case of cautious optimism. Nephrol Dial Transplant. 2016;31(11):1755–8.
Wu M, Gu J, Mei S, Xu D, Jing Y, Yao Q, et al. Resveratrol delays polycystic kidney disease progression through attenuation of nuclear factor κB-induced inflammation. Nephrol Dial Transplant. 2016;31(11):1826–34.
Finkel T, Deng C-X, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–91.
Yuan J, Minter-Dykhouse K, Lou Z. A c-Myc–SIRT1 feedback loop regulates cell growth and transformation. J Cell Biol. 2009;185(2):203–11.
Zhou X, Fan LX, Sweeney WE, Denu JM, Avner ED, Li X. Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J Clin Invest. 2013;123(7):3084–98.
Zhang H-N, Li L, Gao P, Chen H-Z, Zhang R, Wei Y-S, et al. Involvement of the p65/RelA subunit of NF-κB in TNF-α-induced SIRT1 expression in vascular smooth muscle cells. Biochem Biophys Res Commun. 2010;397(3):569–75.
Luo J, Nikolaev AY, Imai S-I, Chen D, Su F, Shiloh A, et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107(2):137–48.
Funding
This work was funded by Fundamental Research Grant Scheme (FRGS) [Grant number: FP086-2019A; reference code: FRGS/1/2019/SKK08/UM/02/10].
Author information
Authors and Affiliations
Contributions
Wrote or contributed to the writing of manuscript: RM, SKL, KCO, KHC, HCC.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Mahendran, R., Lim, S.K., Ong, K.C. et al. Natural-derived compounds and their mechanisms in potential autosomal dominant polycystic kidney disease (ADPKD) treatment. Clin Exp Nephrol 25, 1163–1172 (2021). https://doi.org/10.1007/s10157-021-02111-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-021-02111-x