Abstract
Background
Completion lymph node dissection (CLND) has long been the standard treatment for stage III melanomas identified as metastasis on the sentinel node (SN-positive). Two major changes occurred in 2017 and 2018, the change in the CLND criteria for SN-positive patients and the approval of several adjuvant therapies could revolutionize such management approach. However, their effects have not been fully investigated on the real-world outcomes of stage III melanoma patients. Therefore, we investigated the impact of these changes on the prognosis of Japanese stage III melanoma patients.
Methods
Totally, 119 stage III, SN-positive melanoma patients were included. They were categorized into those diagnosed as SN-positive between January 2015 and June 2017 (pre-June 2017 group) and between July 2017 and December 2019 (post-July 2017 group). Recurrence-free survival (RFS), overall survival, and prognostic factors were analyzed.
Results
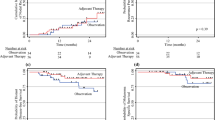
The frequency of patients who received CLND was significantly higher in the pre-June 2017 group (p = 0.001), and those who received adjuvant therapy were significantly higher in the post-July 2017 group (p < 0.001). The 2-year RFS was 50.1% and 68.5% in the pre-June and post-July 2017 groups, respectively (p = 0.049). Cox proportional hazards model analysis for RFS showed that adjuvant therapies reduce the risk of recurrence (hazard ratio 0.37; 95% confidence interval 0.14–0.99; p = 0.047).
Conclusion
Changes in the CLND criteria in SN-positive patients and the approval of adjuvant therapies for stage III melanomas have significantly impacted Japanese melanoma medicine. Adjuvant therapy tended to prolong patient’s RFS while omitting immediate CLND had no significant negative influence on it.



Similar content being viewed by others
References
Gershenwald JE, Scolyer RA, Hess KR et al (2017) Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:472–492
National Comprehensive Cancer Network (2020) Clinical practice guidelines in oncology: cutaneous melanoma. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf. Accessed 15 Apr 2020
Morton DL, Thompson JF, Cochran AJ et al (2014) Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med 370:599–609
Faries MB, Thompson JF, Cochran AJ et al (2017) Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med 376:2211–2222
Leiter U, Stadler R, Mauch C et al (2016) Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol 17:757–767
Long GV, Hauschild A, Santinami M et al (2017) Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 377:1813–1823
Eggermont AMM, Blank CU, Mandala M et al (2018) Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378:1789–1801
Weber J, Mandala M, Del Vecchio M et al (2017) Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377:1824–1835
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Nijhuis AAG, Spillane AJ, Stretch JR et al (2020) Current management of patients with melanoma who are found to be sentinel node-positive. ANZ J Surg 90:491–496
Herb JN, Dunham LN, Ollila DW et al (2020) Use of completion lymph node dissection for sentinel lymph node-positive melanoma. J Am Coll Surg 230:515–524
Orion C, Dinulescu M, Dalac-Rat S et al (2019) Stage III melanoma: sentinel node biopsy, completion lymph node dissection and prospects of adjuvant therapy. A French national survey on current and envisaged practices. Ann Dermatol Vénéréol 147:9–17
Seth R, Messersmith H, Kaur V et al (2020) Systemic therapy for melanoma: ASCO guideline. J Clin Oncol 38:3947–3970
Namikawa K, Aung PP, Milton DR et al (2019) Correlation of tumor burden in sentinel lymph nodes with tumor burden in nonsentinel lymph nodes and survival in cutaneous melanoma. Clin Cancer Res 25:7585–7593
Palve J, Ylitalo L, Luukkaala T et al (2020) Sentinel node tumor burden in prediction of prognosis in melanoma patients. Clin Exp Metastasis 37:365–376
Hu Y, Briggs A, Marchetti MA et al (2020) Cost-benefit implication of gene expression profiling and adjuvant therapy in stage IIIA melanoma. J Am Coll Surg 231:547-554.e1
Farrow NE, Raman V, Williams TP et al (2020) Beasly, adjuvant therapy is effective for melanoma patients with a positive sentinel lymph node biopsy who forego completion lymphadenectomy. Ann Surg Oncol 27:5121–5125
Acknowledgements
This work was partly supported by the National Cancer Center Research and Development Fund (2020-J-3).
Funding
This work was partly supported by the National Cancer Center Research and Development Fund (2020-J-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original publication has been revised for inclusion of Supplementary materials.
Supplementary Information
Below is the link to the electronic supplementary material.



About this article
Cite this article
Ogata, D., Tanese, K., Nakamura, Y. et al. Impact of the changes in the completion lymph node dissection criteria and approval of adjuvant therapies on the real-world outcomes of Japanese stage III melanoma patients. Int J Clin Oncol 26, 2338–2346 (2021). https://doi.org/10.1007/s10147-021-02029-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02029-0