Abstract
Background
Pulmonary complications are common in sickle cell disease (SCD) and can mimic pulmonary embolisms (PEs), leading to potential overuse of computed tomography pulmonary angiography (CTPA). Maximizing the quality of CTPA is essential for its diagnostic accuracy. However, little is known about the positive rate and quality of CTPA in SCD.
Methods
This retrospective case‒control study aimed to determine the positive rate and quality of CTPA studies performed to rule out PE in SCD (HbSS genotype) patients compared to a control group. Logistic regression analysis was used to identify independent factors associated with suboptimal CTPA studies, defined as a mean enhancement of < 210 HU in the pulmonary artery.
Results
The study included 480 patients, consisting of 240 SCD patients and 240 controls. The positive rate of PE was 4.0%, with a similar rate in both SCD patients and the control group (4.2% vs. 3.8%, p = 0.08). However, SCD patients had significantly lower contrast enhancement of the pulmonary artery than the control group (266.1 ± 90.5 HU vs. 342.2 ± 116.1 HU, p < 0.01). Notably, 25.4% of SCD patients had suboptimal scans. The logistic regression model demonstrated that SCD was significantly associated with suboptimal pulmonary arterial contrast enhancement compared to the control group (OR = 4.4; 95% CI: 2.4–8.3).
Conclusions
This study revealed a relatively low positive rate of CTPA in both SCD patients and the control group. However, SCD was significantly associated with suboptimal image quality due to inadequate contrast enhancement of the pulmonary artery. Further research is needed to identify measures that can enhance the quality of CTPA studies in SCD patients and to establish a specific imaging protocol for this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Pulmonary embolism (PE) is a common and potentially life-threatening medical emergency that requires prompt diagnosis and management to minimize associated morbidity and mortality [1]. However, due to its nonspecific clinical presentation, the diagnosis of PE can be challenging and requires a high level of suspicion to pursue an appropriate diagnostic evaluation. Computed tomography pulmonary angiography (CTPA) remains the preferred first-line imaging investigation for evaluating PE due to its high sensitivity and widespread availability [2]. However, concerns have been raised about the overuse of CTPA, which can lead to unnecessary radiation exposure and reactions to contrast media [3, 4]. The positive rate of CTPA is often used as an indicator of appropriate utilization, with the Royal College of Radiologists recommending a positive rate greater than 15.4% [5].
Sickle cell disease (SCD) is an inherited monogenic hematologic disorder with a wide spectrum of complications affecting various organ systems. Cardiopulmonary complications are common and are the leading cause of mortality in SCD [6]. The clinical features of these complications can be indistinguishable from those of PE. Notably, up to 90% of patients with SCD were found to have abnormal pulmonary function tests that could be related to underlying asthma, pulmonary hypertension, pulmonary fibrosis, or pulmonary hemosiderosis [7]. Another challenge is the constant elevation of D-dimer levels in SCD, which limits the use of D-dimer-based diagnostic algorithms [8]. Patients with SCD are also at increased risk for thromboembolic events due to their hypercoagulable state [9]. In particular, the mortality rate from PE in SCD is 5%, making it the second most common pulmonary-related cause of death after pneumonia [10]. As a result, distinguishing PE from other pulmonary conditions can be challenging, and the overuse of CTPA in SCD might be expected, considering that the mortality rate of missed PE can be as high as 30% [11]. However, this issue has not been adequately researched, as only a few studies have examined the positive rate of CTPA for PE in SCD [12, 13]. For instance, Bates et al. [13] found no significant differences in the positive rate of CTPA between patients with SCD and those without SCD. However, this study only focused on patients in the emergency department and included a small sample size of 78 individuals with SCD.
Accurate diagnosis of PE relies on the quality of the CTPA study. Adequate contrast opacification of the pulmonary vasculature is an essential factor impacting diagnostic performance, with the Royal College of Radiologists recommending a mean contrast enhancement greater than 210 Hounsfield units (HU) in the pulmonary artery [14]. However, limited studies have evaluated the quality of CTPA in SCD. For example, a retrospective study by Jensen et al. [15] showed that SCD patients had lower contrast opacification of the pulmonary artery and discussed several modifications to the imaging protocol to enhance scan quality. Nonetheless, some of these modifications, such as increasing contrast volume, can lead to potential adverse effects, such as contrast-induced nephropathy. It may be impractical to incorporate such modifications solely based on the findings of a single study involving only 35 patients with SCD. Additionally, Swift et al. [12] reported contradictory findings, indicating that the image quality of CTPA in SCD was similar to, if not better than, that in the control group.
In the Kingdom of Bahrain, the prevalence of the sickle cell trait was 11%, and the prevalence of SCD was 2.1% according to neonatal screening data in 1985 [16]. Despite extensive efforts in premarital screening and counseling, a more recent study in 2011 indicated that the prevalence of SCD among school students was almost 1.0% [17]. Most previous studies on CTPA in SCD suffered from small sample sizes. Therefore, the present study aims to expand our knowledge of the positive rate and quality of CTPA in SCD in the Kingdom of Bahrain, where the disease is relatively prevalent.
Methods
Study design and settings
After obtaining approval from the ethics committee, a retrospective case‒control study was conducted to investigate the prevalence of PE and evaluate the quality of CTPA studies in terms of contrast opacification of the pulmonary arterial vasculature in patients with SCD compared to a control group of patients without SCD. The study was conducted at the Salmaniya Medical Complex, the largest tertiary hospital in the Kingdom of Bahrain, with a capacity of 1200 beds and 1300 daily visits to the emergency department.
Patient selection
The study enrolled all patients with SCD (HbSS genotype) who underwent CTPA for suspected PE and presented to the hospital between January 1, 2018, and December 31, 2021. Both hospitalized patients and outpatients were included, while patients who were pregnant or under the age of 18 were excluded. Age-matched patients without SCD who underwent CTPA during the same period were randomly selected as a control group.
Data collection
A structured data collection form was developed to extract clinical, laboratory, and radiological information from electronic health records. The clinical data collected included age, sex, comorbidities, and SCD status. The laboratory data collected included hemoglobin (g/dL) and D-dimer (μg/mL) levels. The radiological data collected included the presence of PE, mean contrast opacification of the pulmonary artery, and the main pulmonary artery diameter. The data were collected by coinvestigators who were trained and closely supervised by the principal investigator.
The Charlson Comorbidity Index, a validated tool that quantifies the burden of comorbid conditions in patients and predicts patient mortality and adverse outcomes [18], was calculated. It includes 17 different components and has a range from 0 to 37, with higher scores indicating a greater burden of disease.
Image interpretation
Contrast opacification was assessed by placing a region of interest over two-thirds of the pulmonary artery at its bifurcation. The CTPA study was considered suboptimal if the mean enhancement of the pulmonary artery was less than 210 HU. The pulmonary arterial vasculature was evaluated for filling defects suggestive of PE. The interpretations were compared to the available radiology reports, all of which were approved by board-certified radiologists, and no discrepancies were found. The diameter of the main pulmonary artery was measured at its bifurcation.
Imaging technique
CTPA studies were conducted using a dual-source 128-slice scanner (Siemens, Erlangen, Germany). The bolus tracking technique was used, in which sequential axial slices were obtained at the level of the pulmonary artery until the 100 HU threshold was reached. Multiplanar reconstructions were obtained in the sagittal and coronal planes. The scanning parameters are summarized in Table 1.
Statistical analysis
The data were collected and organized using Microsoft Excel 2021 (Microsoft, Redmond, WA, USA). After ensuring completeness and consistency, the data were analyzed using IBM SPSS for Windows, version 26 (IBM Corp., Armonk, NY, USA). Figures were used to provide an illustrative summary of the data. Categorical variables, presented as percentages and frequency distributions, were compared using the chi-squared test or Fisher’s exact test. The Kolmogorov–Smirnov and Shapiro–Wilk tests determine that the continuous variables were not normally distributed. Hence, continuous variables, presented as medians and interquartile ranges (IQR), were compared using the Mann–Whitney U test. Multivariable binary logistic regression analysis was conducted to identify the independent factors associated with suboptimal quality of CTPA. Candidate variables were selected based on medical literature and bivariate analyses. Odds ratios (ORs) with 95% confidence intervals (CIs) were estimated using the full model fit, and the results were reported in comparison with the designated reference group. The goodness-of-fit of the model was evaluated using the Omnibus and Hosmer–Lemeshow tests. The significance level was defined as α = 0.05.
Results
Patient characteristics
As shown in Table 2, the study enrolled 480 patients, including 240 patients with SCD and a similar number of control patients. There were 250 (52.1%) male and 230 (47.9%) female patients. The median age of the patients was 39.0 years (IQR: 31.0–50.0 years). No significant differences in age, sex, or body mass index were found between the SCD and control groups (p = 0.88). Furthermore, the Charlson comorbidity index scores were comparable in both groups (p = 0.27). Only 5 (1.0%) patients had a history of venous thromboembolic events.
D-dimer level measurements were performed in half of the patient population (50.8%). The median D-dimer level in patients with SCD was significantly higher than that in the control group (4.9 μg/mL vs. 2.7 μg/mL; p < 0.01). However, no significant difference was observed in the proportion of patients with elevated D-dimer levels between the SCD and control groups (95.8% vs. 90.5%; p = 0.11).
Findings of CTPA studies
Table 3 summarizes the positive rate of CTPA according to age, sex, and SCD. Among the 480 patients, 19 had PE, yielding an overall positive rate of 4.0%. Patients with SCD had a positive rate similar to that of the control group (4.2% vs 3.8%; p = 0.08). Additionally, no significant difference was observed in the positive rate based on age and sex (p > 0.05). Among the 220 patients with elevated D-dimer levels, 7 (3.2%) had PE. The median diameter of the main pulmonary artery was 27.7 mm. There was a statistically significant difference in the median diameter of the main pulmonary artery between the SCD and control groups (28.3 mm vs. 27.0 mm; p < 0.01).
Quality of CTPA studies
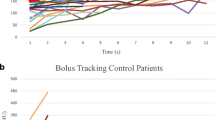
In total, 79 (16.5%) patients had suboptimal contrast opacification of the pulmonary artery in CTPA studies (Figs. 1 and 2). The mean contrast opacification of the pulmonary artery was 304.2 ± 110.7 HU. However, patients with SCD had a significantly lower contrast opacification of the pulmonary artery than those in the control group (266.1 ± 90.5 HU vs. 342.2 ± 116.1 HU; p < 0.01). This difference is further illustrated in Fig. 3.
Axial CT pulmonary angiography images in the thromboembolism-specific window of a 44-year-old man with sickle cell disease and recent recovery from COVID-19 are presented. The images show inadequate contrast enhancement in the pulmonary artery of 156 HU (panel A) and a faint filling defect (arrow) within the right interlobar pulmonary artery consistent with pulmonary embolism (panel B)
Axial CT pulmonary angiography images in the thromboembolism-specific window of a 20-year-old woman without sickle cell disease after recovery from cardiac arrest are presented. The images show optimal contrast enhancement in the pulmonary artery of 788 HU (panel A) and clear filling defects (arrows) within the main pulmonary arteries bilaterally consistent with pulmonary embolism (panel B)
Patients with SCD were four times more likely to have suboptimal contrast opacification of the pulmonary artery on CTPA than those in the control group. In particular, 25.4% of SCD patients had suboptimal scans. Female patients had a lower suboptimal scan rate than their male counterparts (10.4% vs 22.0%; p < 0.05). No significant differences were found in the proportion of suboptimal scans according to age group (p > 0.05) (Table 4).
Multivariable binary logistic regression analysis was conducted to identify the independent factors associated with suboptimal CTPA studies. The model revealed that women were 50% less likely (OR = 0.5; 95% CI: 0.3–0.8) to have suboptimal scans. Patients with SCD were 4.4 times more likely (OR = 4.4; 95% CI: 2.4–8.3) to have suboptimal scans than those in the control group (Table 5).
Discussion
The present study aimed to investigate the positive rate of CTPA and to quantitatively assess the quality of contrast opacification of the pulmonary arterial vasculature. The positive rate for PE was found to be comparable to that of the control group. However, patients with SCD had a significantly higher proportion of suboptimal scans.
The overall positive rate of CTPA was markedly below the standard recommended by the Royal College of Radiologists. Previous studies have reported widely divergent positive rates of CTPA between different countries and institutions, ranging from approximately 1 to 34% [19,20,21]. Several factors have been linked to a lower positive rate of CTPA. Importantly, clinical scoring systems, such as the Wells criteria for PE, are not routinely applied despite the recommended guidelines [22, 23]. The practice of defensive medicine could be a relevant underlying factor. Given the large number of studies that have demonstrated an increased risk of thromboembolic events in SCD [24,25,26], a higher positive rate of CTPA in SCD was expected. However, this study showed that the SCD group had a positive rate similar to that of the control group. This finding could be related to the lower threshold to perform imaging studies in SCD, leading to an increased number of negative scans. Furthermore, the higher proportion of suboptimal scans in SCD can result in a higher number of false-negative scans, as most PEs in SCD involve the peripheral vasculature [27, 28], which requires high-quality scans for detection. The positive rate of CTPA in the control group of this study may not be representative of the general population of patients who underwent CTPA, as they were matched in age to the SCD group, which has a younger age profile [28].
The findings revealed that SCD patients had a higher tendency to have suboptimal scans due to inadequate contrast opacification of the pulmonary arterial vasculature. The results are consistent with the study by Jensen et al. [15], who found that almost 75% of SCD patients had a mean contrast enhancement of less than 250 HU in the pulmonary artery compared to 32% of patients in the control group, although both groups had a similar contrast volume and infusion rate. The reason for the inadequate contrast opacification of the pulmonary arterial vasculature in SCD remains unclear. However, that study postulated that altered cardiovascular dynamics in SCD seem to influence the contrast dynamics [15].
Various measures were recommended to mitigate the effects of inadequate contrast opacification of the pulmonary arterial vasculature in SCD. First, increasing the infusion rate with the use of fenestrated catheters may improve image quality without increasing the iodine load delivered [29]. Second, lowering kVp can improve image quality but at the expense of increasing image noise [30]. Third, obtaining the scan during the expiratory phase can eliminate transient enhancement artifacts [31]. However, future research is needed to assess image quality in SCD after implementing some of these strategies.
Ventilation-perfusion scintigraphy is a viable imaging option, particularly for stable patients with normal chest radiographs [32]. Compared to CTPA studies, ventilation-perfusion scintigraphy exposes patients to less radiation and does not require intravenous contrast administration [33]. This is especially critical for patients with SCD, who are typically young [28]. Up to 10% of SCD patients who undergo imaging studies for PE require a second study within 90 days of the initial study [32], further emphasizing the need to minimize radiation exposure.
While this study is the largest to date to investigate the positive rate and image quality of CTPA studies in patients with SCD, it is not without limitations. The retrospective nature of the study is an important limitation. For instance, some patients may have undergone a scan with certain modifications to the standard scanning protocol (e.g., different catheter sizes), and this information was not readily available. Additionally, the lack of subjective assessment of the scans is another limitation, as other artifacts may negatively impact the diagnostic accuracy of the scan, despite adequate contrast opacification of the pulmonary arterial vasculature. There are several types of artifacts that can have a negative impact on the quality of CTPA studies. These include motion artifacts caused by breathing or other movements during the scan, transient interruption of contrast, streak artifacts that can be related to the contrast material in the superior vena cava, and inadequate contrast enhancement of the pulmonary vasculature adjacent to areas of parenchymal disease [34].
Conclusions
This study found a relatively low positive rate of CTPA in both SCD patients and the control group. However, SCD was significantly associated with suboptimal image quality due to inadequate pulmonary arterial contrast enhancement. Further research is needed to explore potential strategies for enhancing the quality of CTPA in SCD patients. Such investigations may pave the way for developing a customized imaging protocol for this patient population.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI :
-
Confidence interval
- CTPA :
-
Computed tomography pulmonary angiography
- HU :
-
Hounsfield unit
- IQR :
-
Interquartile range
- OR :
-
Odds ratio
- PE :
-
Pulmonary embolism
- SCD :
-
Sickle cell disease
References
Wendelboe AM, Raskob GE (2016) Global burden of thrombosis: epidemiologic aspects. Circ Res 118(9):1340–1347
Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP et al (2019) 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J 54(3):1901647
Wang RC, Miglioretti DL, Marlow EC, Kwan ML, Theis MK, Bowles EJA et al (2020) Trends in imaging for suspected pulmonary embolism across US health care systems, 2004 to 2016. JAMA Netw Open 3(11):e2026930
Hassan A, Al Dandan O, Awary K, Bukhamsin B, Bukhamseen R, Alzaki A et al (2021) Determinants of time-to-disposition in patients who underwent CT for pulmonary embolism: a retrospective study. BMC Emerg Med 21(1):118
Quigley A, Brown K. Appropriateness of usage of computed tomography pulmonary angiography (CTPA) investigation of suspected pulmonary embolism. Available at: https://www.rcr.ac.uk/audit/appropriateness-usagecomputed- tomography-pulmonary-angiography-ctpa-investigation-suspected. Accessed 21 Mar 2023
Fitzhugh CD, Lauder N, Jonassaint JC, Telen MJ, Zhao X, Wright EC et al (2010) Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol 85(1):36–40
Klings ES, Wyszynski DF, Nolan VG, Steinberg MH (2006) Abnormal pulmonary function in adults with sickle cell anemia. Am J Respir Crit Care Med 173(11):1264–1269
Fakunle EE, Eteng K, Shokunbi WA (2012) DD dimer levels in patients with sickle cell disease during bone pain crises and in the steady state. Pathol Laborat Med Int 4:21–25
Noubiap JJ, Temgoua MN, Tankeu R, Tochie JN, Wonkam A, Bigna JJ (2018) Sickle cell disease, sickle trait and the risk for venous thromboembolism: a systematic review and meta-analysis. Thromb J 16(1):27
Hamideh D, Alvarez O (2013) Sickle cell disease related mortality in the United States (1999–2009). Pediatr Blood Cancer 60(9):1482–1486
Belohlavek J, Dytrych V, Linhart A (2013) Pulmonary embolism, part I: epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol 18(2):129–138
Swift CA, Nance JW, Collins H, Ravenel JG (2019) Incidence of pulmonary embolism in sickle cell anemia patients undergoing computed tomography pulmonary angiography in the emergency department. J Thorac Imaging 34(5):W125–W126
Bates DDB, Liu Z, Gibbons J, LeBedis CA, Holalkere NS (2019) Sickle cell disease and venous thromboembolism: a retrospective comparison of the rate of positive CT pulmonary angiography in the emergency department. Eur J Radiol 110:256–259
Muller M, Beattie A. Adequate contrast enhancement of CT pulmonary angiograms. Available at: https://www.rcr.ac.uk/audit/adequate-contrast-enhancement-ct-pulmonary-angiograms. Accessed 21 Mar 2023
Jensen J, Lin T, Fishman EK, Johnson PT (2017) Pulmonary CTA in sickle cell patients: quantitative assessment of enhancement quality. Emerg Radiol 24(6):667–674
Al AS (2005) Campaign to control genetic blood diseases in Bahrain. Commun Genet 8(1):52–55
Al Arrayed SS (2011) Prevalence of abnormal hemoglobins among students in Bahrain: a ten-year study. Bahrain Med Bull 33(1):19–21
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Dhakal P, Iftikhar MH, Wang L, Atti V, Panthi S, Ling X et al (2019) Overutilisation of imaging studies for diagnosis of pulmonary embolism: are we following the guidelines? Postgrad Med J 95(1126):420–424
Alshumrani G, Al Bshabshe A, Mousa WF (2021) Diagnostic yield of CT pulmonary angiography for pulmonary embolism in clinically suspected patients. Medicine (Baltimore) 100(22):e26213
Alsaif HS, Hassan A, AlSheikh M, Al-Sulaibeekh A, Alnasr A, Alzaki A et al (2020) Predictors of positive computed tomography pulmonary angiography results. Emerg Radiol 27(5):503–511
Hsu N, Soo Hoo GW (2020) Underuse of clinical decision rules and d-dimer in suspected pulmonary embolism: a nationwide survey of the veterans administration healthcare system. J Am Coll Radiol 17(3):405–411
Al Dandan O, Hassan A, Alnasr A, Al Gadeeb M, AbuAlola H, Alshahwan S et al (2020) The use of clinical decision rules for pulmonary embolism in the emergency department: a retrospective study. Int J Emerg Med 13(1):23
Novelli EM, Huynh C, Gladwin MT, Moore CG, Ragni MV (2012) Pulmonary embolism in sickle cell disease: a case-control study. J Thromb Haemost 10(5):760–766
Seaman CD, Yabes J, Li J, Moore CG, Ragni MV (2014) Venous thromboembolism in pregnant women with sickle cell disease: a retrospective database analysis. Thromb Res 134(6):1249–1252
Naik RP, Streiff MB, Haywood C Jr, Segal JB, Lanzkron S (2014) Venous thromboembolism incidence in the cooperative study of sickle cell disease. J Thromb Haemost 12(12):2010–2016
Gopalsamy SN, El Rassi F, McLemore ML (2017) Pulmonary embolism in sickle cell disease: retrospective analysis of the use and yield of radiographic studies. Blood 130:4792
Al Dandan O, Hassan A, AbuAlola H, Alzaki A, Alwaheed A, Alalwan M et al (2020) Clinical and imaging profiles of pulmonary embolism: a single-institution experience. Int J Emerg Med 13(1):47
Johnson PT, Christensen GM, Fishman EK (2014) I.v. contrast administration with dual source 128-MDCT: a randomized controlled study comparing 18-gauge nonfenestrated and 20-gauge fenestrated catheters for catheter placement success, infusion rate, image quality, and complications. AJR Am J Roentgenol. 202(6):1166–1170
Leithner D, Gruber-Rouh T, Beeres M, Wichmann JL, Mahmoudi S, Martin SS et al (2018) 90-kVp low-tube-voltage CT pulmonary angiography in combination with advanced modeled iterative reconstruction algorithm: effects on radiation dose, image quality and diagnostic accuracy for the detection of pulmonary embolism. Br J Radiol 91(1088):20180269
Mortimer AM, Singh RK, Hughes J, Greenwood R, Hamilton MC (2011) Use of expiratory CT pulmonary angiography to reduce inspiration and breath-hold associated artefact: contrast dynamics and implications for scan protocol. Clin Radiol 66(12):1159–1166
Tivnan P, Billett HH, Freeman LM, Haramati LB (2018) Imaging for pulmonary embolism in sickle cell disease: a 17-year experience. J Nucl Med 59(8):1255–1259
Schembri GP, Miller AE, Smart R (2010) Radiation dosimetry and safety issues in the investigation of pulmonary embolism. Semin Nucl Med 40(6):442–454
Wittram C (2007) How I do it: CT pulmonary angiography. Am J Roentgenol 188(5):1255–1261
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were implemented in accordance with the Declaration of Helsinki, and the study was approved by the Ethics Review Committee. The requirement for informed consent was waived by the Ethics Committee of the Salmaniya Medical Complex in Bahrain because of the retrospective nature of the study, and no personal identification data were requested or stored.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassan, A., Taleb, M., Hasan, W. et al. Positive rate and quality assessment of CT pulmonary angiography in sickle cell disease: a case‒control study. Emerg Radiol 30, 209–216 (2023). https://doi.org/10.1007/s10140-023-02126-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-023-02126-9