Abstract
Detection of SARS-CoV-2 RNA in serum, viremia, has been linked to disease severity and outcome. The kinetics of viremia in patients receiving remdesivir has not been thoroughly studied and could help predict treatment response and outcome. We investigated the kinetics of SARS-CoV-2 viremia and factors associated with baseline viremia, viral clearance and 30-day mortality in patients receiving remdesivir. An observational study including 378 hospitalised patients (median age 67 years, 67% male) sampled with serum SARS-CoV-2 RT-PCR within ± 24 h of initiation of remdesivir treatment. Baseline viremia was present in 206 (54%) patients with a median Ct value of 35.3 (IQR = 33.3–37.1). In patients with baseline viremia, the estimated probability of viral clearance was 72% by day 5. Ct values decreased significantly during remdesivir treatment for viremic patients, indicating an increase in viral load. In total, 44 patients (12%) died within 30 days, and mortality was significantly associated with viremia at baseline (OR = 2.45, p = 0.01) and lack of viral clearance by day 5 (OR = 4.8, p = < 0.01). Viral clearance was not associated with any individual risk factor. Viremia appears to be a prognostic marker before and during remedesivir treatment. The resolution of viremia was similar to patients not receiving remdesivir in other studies, and the decrease in Ct values during treatment questions the antiviral capacity of remdesivir in vivo. Prospective studies are warranted to confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Detection of SARS-CoV-2 RNA by real-time PCR (RT-PCR) in serum, viremia, has been reported to correlate with disease severity, ICU admission and mortality, and may be used as a predictor for the clinical outcome [1-5]. Therefore, viremia could be a marker of uncontrolled viral replication and play a significant role in the pathogenesis of severe COVID-19. Further, disseminated viral particles from the lungs could drive the dysregulated inflammatory host response seen in critical COVID-19 patients [6].
Remdesivir is a nucleoside analogue (GS-5734) that inhibits RNA polymerase activity and was initially developed for the Ebola virus. Remdesivir was the first antiviral drug approved for the treatment of COVID-19 [7] and has demonstrated potent antiviral activity against the SARS-CoV-2 virus in vitro, including emerging variants of concern [8-10]. Despite promising data from in vitro studies and early primate models [11], subsequent clinical trials have reported conflicting results. For example, the ACTT-1 and Spinner trials [12, 13] did not demonstrate any survival benefit or impact of remdesivir on viral shedding. However, in both trials, the median symptom duration at the treatment start exceeded seven days. At this time, viral replication is expected to be low, limiting the use of an antiviral agent. The WHO-sponsored SOLIDARITY trial [14], the most extensive study to date, demonstrated a slight benefit in mitigating disease progression and improving survival in patients without mechanical ventilation. However, the duration of symptoms at the treatment start was not reported.
Growing evidence shows that remdesivir does not contribute to viral clearance in the upper respiratory tract [15-17]. Nevertheless, viremia may be a better marker of clinically relevant viral replication due to the reported association with disease progression and mortality [1, 18]. There is a lack of studies on the kinetics of SARS-CoV-2 viremia in patients receiving remdesivir. This study aims to bridge this gap by investigating retrospectively the prevalence and kinetics of SARS-CoV-2 RNA in serum among COVID-19 patients receiving remdesivir. A secondary aim of this study was to identify factors associated with baseline viremia, lack of viral clearance and mortality at 30 days.
Materials and methods
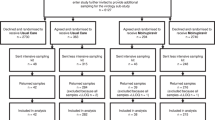
This retrospective cohort study included all hospitalised COVID-19 patients ≥ 18 years receiving remdesivir between 1 July 2020 and 30 April 2021 at Akademiska University Hospital, a tertiary care hospital in Uppsala and Västerås Hospital, a regional hospital in Västerås, Sweden. The inclusion criteria were the measurement of serum SARS-CoV-2 by RT-PCR within + / − 24 h of initiation of remdesivir treatment. At the study centres, serum SARS-CoV-2 RT-PCR testing was recommended at the initiation of remdesivir to evaluate the virological response and was typically performed every 2–3 days at the attending physician’s discretion. Serum from peripheral blood was collected from the patients, and viral kinetics was determined by sequential serum SARS-CoV-2 RT-PCR testing.
At the Department of Microbiology at the Karolinska University Hospital, Huddinge, Stockholm, the following commercial techniques approved for diagnosing SARS-CoV-2 were used: cobas SARS-CoV-2 assay (Roche, Pleasanton, California), targeting the Envelope (E) and Open Reading Frame (ORF) 1 gene [19] or NeuMoDx SARS-CoV-2 assay (NeuMoDx, Ann Arbor, Michigan) targeting non-structural protein 2 (Nsp 2), RNA-dependent RNA polymerase (RdRp) and nucleocapsid (N) genes. In addition, a validated in-house method targeting the RdRp and E-genes was utilised. At the Department of Microbiology at Akademiska University Hospital, Uppsala, a validated in-house method targeting the N1 and E genes was used. All analyses were performed per the manufacturer’s instruction and under standard clinical practice. Cycle threshold (Ct) values were used as a surrogate marker of SARS-CoV-2 viral load. In cases of multiple Ct values, the target gene with the lowest Ct value was chosen and used for subsequent samples. Ct-values > 40 were considered negative and were not included in analyses involving Ct values. Ct values over time were analysed with linear regression.
The primary analyses were viremia at the start of remdesivir treatment, viral clearance, and mortality at 30 days. Clinical and physiological data and baseline characteristics were acquired from the patient’s medical records and reported as medians, interquartile range, frequency, and percentage for continuous and categorical variables, respectively. The risk factors associated with severe COVID-19 were adopted from the Center for Disease Control and Prevention [20].
Statistical analysis
STATA version 16.1 (StataCorp, Texas, USA) software was used for the statistical analyses. Baseline characteristics were assessed using the Shapiro-Wilk test for normality, and results were presented as median and interquartile range (IQR) when normality could be rejected at a 0.05 significance level. Logistic regression was used to analyse risk factors associated with baseline viremia and 30-day mortality and presented as odds ratios with an alpha significance level of 0.01 to account for multiple testing. Viral clearance was estimated using the Kaplan-Meier survival analysis. Cox regression was utilised to investigate the impact of a set of clinically relevant predictor variables on the time to achieve negative viremia, presented as hazard ratios with an alpha significance level of 0.01. Ct-values over time were analysed using linear regression.
The study was performed following the guidelines of the Declaration of Helsinki and approved by the Swedish Ethical Review Board, Stockholm (Dnr 2021-01878). The Swedish Ethical Review Board waived the need for informed consent.
Results
Remdesivir treatment was initiated in 435 patients during the study period, of whom 378 had a measurement of serum SARS-CoV-2 within ± 24 h of remdesivir treatment start and were included in the study. The baseline characteristics of the study population, including 254 (67%) men and 124 (33%) women, are presented in Table 1. The median age was 67 years, and the median BMI was 28 kg/m2. The most common risk factors were hypertension (n = 240, 63%), BMI > 30 kg/m2 (n = 166, 44%) and diabetes mellitus (n = 116, 31%). The patients had a median symptom duration of seven days (IQR = 5–8 days) at the start of remdesivir therapy, and 338 (89%) patients required supplemental oxygen at the start of remdesivir treatment.
Baseline viremia was detected in 206 (54%) patients with a median Ct value of 35.3 (IQR = 33.3–37.1). There was a strong association between baseline viremia and CRP > 100 mg/L (OR 1.9, p = < 0.01) and supplemental oxygen (OR 5.66, p = < 0.01), but no association was found with symptom duration. The median duration of remdesivir treatment was five days (IQR = 4–5 days).
A second SARS-CoV-2 RT-PCR was performed in 271 (72%) patients, and out of them, 76 (20%) patients were sampled thrice. In patients with baseline viremia, the Kaplan-Meier probability of viral clearance was 72% by day 5 (Fig. 1). The median duration of symptoms at viral clearance was ten days (IQR = 8–11 days). There were no significant associations between viral clearance and individual risk factors (Table 2). Ct values decreased significantly throughout remdesivir treatment (R2 = 0.03, p = 0.01) (Fig. 2).
We also report the death of 44 patients (12%) within 30 days. Mortality at 30 days was significantly associated with age > 65 years (OR = 49.1, p = < 0.01), viremia at baseline (OR = 2.45, p = 0.01) and cardiovascular disease (OR = 3.16, p = < 0.01) (Table 3). Viremia at baseline remained a significant risk factor in a multiple logistic regression model, including age, sex and at least one risk factor (OR = 2.8, p < 0.01).
In total, 207 (55%) patients received dexamethasone, and 174 (46%) received antibiotic therapy. The median length of hospital stay was ten days.
Discussion
This observational retrospective study is the first to investigate SARS-CoV-2 viremia in patients treated with remdesivir. Remdesivir is still widely used partly due to the extensive drug interactions associated with the newer antiviral nirmatrelvir/ritonavir.
We found viremia to be an independent prognostic marker in remdesivir-treated patients due to the strong association with mortality. The association was unaffected when included in a multiple regression model including age, sex and at least one risk factor.
The probability of viral clearance by the end of remdesivir treatment (day 5) was only 72%, and the lack of viral clearance before day five was significantly associated with increased mortality. The median symptom duration at viral clearance was ten days, similar to a cohort study by Hagman et al. [1], where 24% (99/417) of hospitalised patients were viremic when sampled within three of admission and with a medium symptom duration of ten days. In another study by Ram-Mohan et al. [3], 52% (14/27) of COVID-patients had undetectable plasma SARS-CoV-2 RNA within ten days from the onset of symptoms, indicating resolution of viremia in a majority of patients within ten days regardless of remdesivir treatment.
In addition, we observed a significant reduction in Ct values indicating increased viral load among viremic patients throughout the treatment, which questions the antiviral capacity of remdesivir in-vivo. However, only patients that remained viremic were included in the analysis, which amplifies the effect. Two other studies support the lack of antiviral effect in vivo. Firstly, in the DisCoVeRy study [21], Ader et al. did not find any difference in the viral load in nasal swabs between the remdesivir group and the standard of care group, though a late administration of the drug (median nine days after onset of symptoms) partly weakens the result. Secondly, a recent study by Sourander et al. [15], investigated the kinetics of SARS-CoV-2 RNA in nasopharynx/throat swabs and found no significant difference in viral clearance between remdesivir-treated patients and controls.
Real-life data are vital to understanding how remdesivir is implemented under normal circumstances, which may differ substantially from clinical trials. We observed a median duration of symptoms of seven days at the start of treatment, at the upper limit of the Swedish national treatment guidelines and the WHO recommendations stating, “Remdesivir should be administered as soon as possible after the onset of symptoms, ideally within seven days [22, 23]. The treatment duration for the absolute majority of patients was five days, as recommended by the guidelines.
Baseline viremia was present in only 54% of the patients, and the high median Ct value of 35.3 is consistent with reports of the highest viral titres early in the first week of illness. We found no significant associations between baseline viremia and symptom duration. This discrepancy may partly be explained by a close clustering of most patients around a symptom duration of seven days, limiting the statistical power to detect a time-dependent association.
Previous studies have reported a positive association between viremia and disease severity [18, 24, 25]. In a prospective case series study involving 57 hospitalised COVID-19 patients, persistent viremia was found to be correlated with adverse clinical outcomes, potentially serving as an identifier for patients at risk [26]. Thus, an uncontrolled viral replication may induce and/or exacerbate a dysregulated host response, including cytokine storm, in patients with severe COVID-19. This hypothesis is supported by a study conducted by Rovito et al., which demonstrated increased pro-inflammatory cytokines, diminished activated T-cells, and reduced functional SARS-CoV-2-specific immune responses in viremic patients [27]. Another hypothesis is that inflammation-induced capillary leakage in the lungs may lead to the translocation of viral particles into the systemic circulation, which is detected by the RT-PCR analysis [28]. Viremia may reflect the disease severity but may not exacerbate the disease phenotype in these patients. We found an association between baseline viremia and 30-day mortality and a robust association between CPR levels above 100 mg/L and baseline viremia, consistent with both of these hypotheses.
Overall, a large set of variables were used to analyse the outcomes of 30-day mortality and viral clearance. To account for multiple testing and the risk of false positives, the significance level was lowered to an alpha significance level of 0.01.
The strength of our study is the inclusion of a relatively large number of patients receiving remdesivir in a real-life setting. Sequential viremia testing was available in most patients, 72% were tested twice, but only 20% were tested thrice. The co-administration of other COVID-19 medications, for example, dexamethasone, was present in 55% of the cases, further strengthening the clinical translatability of the results. However, due to the observational design of the study, it was not possible to evaluate the potential effects of steroids on viremia.
Limitations of our study include the retrospective design and lack of a control group restricting conclusions regarding the treatment effects. Also, testing was not conducted at standardised time points, limiting the number of patients where sequential testing was available. Viremia testing was conducted based on clinical judgement and, therefore, subject to confounding. For example, viral clearance could be underestimated due to missing follow-up viremia testing, potentially biased towards not testing clinically improving patients. The study design is not mechanistic, so any causal inference of the predictive capability of viremia should be cautiously interpreted. The majority of patients in our study were included from a period when SARS-CoV-2 vaccination was unavailable, and until the end of the study, vaccination rates remained low. As a result, the impact of immunisation on our findings is highly unlikely. However, the absence of vaccination status information for our cohort represents a limitation of our study.
Various PCR methods were used throughout the study and may not represent a single standardised method. Further, Ct values can differ significantly between assays and target genes, and drawing a solid conclusion regarding the evolution of Ct values in our data is difficult. We used Ct values as a surrogate marker for viral load but may not accurately represent the true viral load as some studies did not find a strong association between the Ct value and clinical markers or infectivity [29, 30]. Finally, patients included in the study were predominantly infected with the original strain and the alfa and delta variants of concern, and the results could potentially not be valid for current omicron variants.
In conclusion, SARS-CoV-2 serum RT-PCR appears to be a prognostic disease marker before and during remdesivir treatment. The resolution of viremia in remdesivir-treated patients was similar to untreated patients in other studies questioning the antiviral efficacy of remdesivir in treating COVID-19 infection. Clinical implications of the study include questioning the use of remdesivir in younger patients without baseline viremia, where the overall mortality appears to be low. Clinical prospective studies are warranted to investigate the effect of remdesivir on the kinetics of SARS-CoV-2 RT-PCR in serum.
Data availability
The data presented in this study are available from the corresponding author upon request, and the data are not publicly available due to ethical restrictions.
References
Hagman K, Hedenstierna M, Rudling J, Gille-Johnson P, Hammas B, Grabbe M et al (2022) Duration of SARS-CoV-2 viremia and its correlation to mortality and inflammatory parameters in patients hospitalised for COVID-19: a cohort study. Diagn Microbiol Infect Dis 102(3):115595. https://doi.org/10.1016/j.diagmicrobio.2021.115595
Jacobs JL, Bain W, Naqvi A, Staines B, Castanha PMS, Yang H et al (2022) Severe Acute Respiratory Syndrome Coronavirus 2 Viremia Is Associated With Coronavirus Disease 2019 Severity and Predicts Clinical Outcomes. Clin Infect Dis 74(9):1525–1533. https://doi.org/10.1093/cid/ciab686
Ram-Mohan N, Kim D, Zudock EJ, Hashemi MM, Tjandra KC, Rogers AJ et al (2022) SARS-CoV-2 RNAemia Predicts Clinical Deterioration and Extrapulmonary Complications from COVID-19. Clin Infect Dis 74(2):218–226. https://doi.org/10.1093/cid/ciab394
Li Y, Schneider AM, Mehta A, Sade-Feldman M, Kays KR, Gentili M, et al (2021) SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. J Clin Invest 131(13). https://doi.org/10.1172/JCI148635
Monchi M, Bruneau T, Jochmans S, Veyer D, Pitsch A, Ellrodt O et al (2022) Association of high SARS-CoV-2 RNAemia with diabetes and mortality in critically ill COVID-19 patients. iScience 25(5):104075. https://doi.org/10.1016/j.isci.2022.104075
Trougakos IP, Stamatelopoulos K, Terpos E, Tsitsilonis OE, Aivalioti E, Paraskevis D et al (2021) Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalised treatments that target COVID-19 clinical complications. J Biomed Sci 28(1):9. https://doi.org/10.1186/s12929-020-00703-5
Brown AJ, Won JJ, Graham RL, Dinnon KH 3rd, Sims AC, Feng JY et al (2019) Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res 169:104541. https://doi.org/10.1016/j.antiviral.2019.104541
Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY et al (2020) Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res 178:104786. https://doi.org/10.1016/j.antiviral.2020.104786
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M et al (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30(3):269–271. https://doi.org/10.1038/s41422-020-0282-0
Vangeel L, Chiu W, De Jonghe S, Maes P, Slechten B, Raymenants J et al (2022) Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res 198:105252. https://doi.org/10.1016/j.antiviral.2022.105252
Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J et al (2020) Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 585(7824):273–276. https://doi.org/10.1038/s41586-020-2423-5
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC et al (2020) Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med 383(19):1813–1826. https://doi.org/10.1056/NEJMoa2007764
Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A et al (2020) Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomised Clinical Trial. JAMA 324(11):1048–1057. https://doi.org/10.1001/jama.2020.16349
(2022) Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. The Lancet 399(10339):1941–53. https://doi.org/10.1016/S0140-6736(22)00519-0
Sourander B, Andersson L-M, Brink M, Yilmaz A, Sundell N, Marklund E et al (2022) No effect of remdesivir or betamethasone on upper respiratory tract SARS-CoV-2 RNA kinetics in hospitalised COVID-19 patients: a retrospective observational study. Infect Dis 54(10):703–712. https://doi.org/10.1080/23744235.2022.2081716
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y et al (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395(10236):1569–1578. https://doi.org/10.1016/s0140-6736(20)31022-9
Barratt-Due A, Olsen IC, Nezvalova-Henriksen K, Kåsine T, Lund-Johansen F, Hoel H et al (2021) Evaluation of the Effects of Remdesivir and Hydroxychloroquine on Viral Clearance in COVID-19: A Randomised Trial. Ann Intern Med 174(9):1261–1269. https://doi.org/10.7326/m21-0653
Hagman K, Hedenstierna M, Gille-Johnson P, Hammas B, Grabbe M, Dillner J et al (2020) Severe Acute Respiratory Syndrome Coronavirus 2 RNA in Serum as Predictor of Severe Outcome in Coronavirus Disease 2019: A Retrospective Cohort Study. Clin Infect Dis 73(9):e2995–e3001. https://doi.org/10.1093/cid/ciaa1285
Martín Ramírez A, Zurita Cruz ND, Gutiérrez-Cobos A, Rodríguez Serrano DA, González Álvaro I, Roy Vallejo E et al (2022) Evaluation of two RT-PCR techniques for SARS-CoV-2 RNA detection in serum for microbiological diagnosis. J Virol Methods 300:114411. https://doi.org/10.1016/j.jviromet.2021.114411
Yek C, Warner S, Wiltz JL, Sun J, Adjei S, Mancera A et al (2022) Risk Factors for Severe COVID-19 Outcomes Among Persons Aged ≥18 Years Who Completed a Primary COVID-19 Vaccination Series - 465 Health Care Facilities, United States, December 2020-October 2021. MMWR Morb Mortal Wkly Rep 71(1):19–25. https://doi.org/10.15585/mmwr.mm7101a4
Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D et al (2022) Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis 22(2):209–221. https://doi.org/10.1016/S1473-3099(21)00485-0
World Health O (2022) Therapeutics and COVID-19: living guideline, 22 April 2022. World Health Organization, Geneva
infektionsläkarföreningen S. Nationellt vårdprogram för misstänkt ochbekräftad covid-19. https://infektion.net/wp-content/uploads/2022/06/nationellt-vardprogram-covid-version-4-0.pdf. Accessed June 2022
Bermejo-Martin JF, González-Rivera M, Almansa R, Micheloud D, Tedim AP, Domínguez-Gil M et al (2020) Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit Care 24(1):691. https://doi.org/10.1186/s13054-020-03398-0
Cardeñoso Domingo L, Roy Vallejo E, Zurita Cruz ND, Chicot Llano M, Ávalos Pérez-Urria E, Barrios A et al (2022) Relevant SARS-CoV-2 viremia is associated with COVID-19 severity: Prospective cohort study and validation cohort. J Med Virol 94(11):5260–5270. https://doi.org/10.1002/jmv.27989
Zurita-Cruz ND, Martín-Ramírez A, Rodríguez-Serrano DA, González-Álvaro I, Roy-Vallejo E, De la Cámara R et al (2022) Usefulness of real-time RT-PCR to understand the kinetics of SARS-CoV-2 in blood: A prospective study. J Clin Virol 152:105166. https://doi.org/10.1016/j.jcv.2022.105166
Rovito R, Bono V, Augello M, Tincati C, Mainoldi F, Beaudoin-Bussières G et al (2022) Association between SARS-CoV-2 RNAemia and dysregulated immune response in acutely ill hospitalised COVID-19 patients. Sci Rep 12(1):19658. https://doi.org/10.1038/s41598-022-23923-1
Järhult JD, Hultström M, Bergqvist A, Frithiof R, Lipcsey M (2021) The impact of viremia on organ failure, biomarkers and mortality in a Swedish cohort of critically ill COVID-19 patients. Sci Rep 11(1):7163. https://doi.org/10.1038/s41598-021-86500-y
Abdulrahman A, Mallah SI, Alqahtani M (2021) COVID-19 viral load not associated with disease severity: findings from a retrospective cohort study. BMC Infect Dis 21(1):688. https://doi.org/10.1186/s12879-021-06376-1
Platten M, Hoffmann D, Grosser R, Wisplinghoff F, Wisplinghoff H, Wiesmüller G, Schildgen O, Schildgen V (2021) SARS-CoV-2, CT-Values, and infectivity-conclusions to be drawn from side observations. Viruses 13(8):1459. https://doi.org/10.3390/v13081459
Acknowledgements
We want to express our deepest gratitude to the clinical research nurses Angelica Norling, Marie Stenius Svensson and Maria Gren for all the assistance and support in conducting the study.
Funding
Open access funding provided by Karolinska Institute. This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Anders Krifors, Linda Karlsson, Camilla Lorant, Martin Ekman and Paul Skorup. The first draft of the manuscript was written by Anders Krifors, Linda Karlsson and Paul Skorup and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Swedish Ethical Review Board, Stockholm (Dnr 2021-01878) and date of approval 21 April 2021).
Consent to participate
The Swedish Ethical Review Board waived the need for informed consent as the research was retrospective and observational and involved minimal risk to participants.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krifors, A., Karlsson, L., Ekman, M. et al. The kinetics of SARS-CoV-2 viremia in COVID-19 patients receiving remdesivir. Eur J Clin Microbiol Infect Dis 42, 951–958 (2023). https://doi.org/10.1007/s10096-023-04627-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04627-4