Abstract
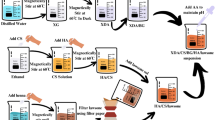
This study used a rabbit model to investigate the osteocompatibility of Si3N4-coated carbon fiber-reinforced polyetheretherketone (CFRP) and hydroxyapatite (HA)-coated CFRP with antibiotics (vancomycin [VCM]) and antithrombotic drugs (polyvinylpyrrolidone [PVP]). HA-coated cylindrical CFRP implants were used as the controls (HA), and HA-coated implants treated with VCM and PVP were prepared (HA-VP) as the test groups; a cylindrical CFRP coated with Si3N4 was also prepared (SiN). Ten implants from each group were randomly inserted into the femoral diaphysis of rabbits. The pull-out test, radiological analysis using micro-computed tomography (µ-CT), and histological analysis were performed. The pull-out strength of the SiN group was lower than that of the HA group. µ-CT analysis revealed that the amount of bone formation around the implant in the SiN group was inferior to that in the HA group. Conversely, the HA-VP group had equivalent pull-out strength and bone formation as analyzed by µ-CT compared to the HA group. In conclusion, the additional surface treatment of the HA-coated CFRP with VCM and PVP provided sufficient bone fixation and formation.






Similar content being viewed by others
References
Abdelaziz H, Rademacher K, Suero EM, Gehrke T, Lausmann C, Salber J, Citak M. The 2018 international consensus meeting minor criteria for chronic hip and knee periprosthetic joint infection: validation from a Single Center. J Arthroplasty. 2020;35:2200–3.
Leonard HA, Liddle AD, Burke Ó, Murray DW, Pandit H. Single- or two-stage revision for infected total hip arthroplasty? A systematic review of the literature. Clin Orthop Relat Res. 2014;472:1036–42.
Kildow BJ, Della-Valle CJ, Springer BD. Single vs 2-stage revision for the treatment of periprosthetic joint infection. J Arthroplasty. 2020;35:S24-30.
Lee WY, Hwang DS, Kang C, Shin BK, Zheng L. Usefulness of prosthesis made of antibiotic-loaded acrylic cement as an alternative implant in older patients with medical problems and periprosthetic hip infections: a 2- to 10-year follow-up study. J Arthroplasty. 2017;32:228–33.
Eto S, Kawano S, Someya S, Miyamoto H, Sonohata M, Mawatari M. First clinical experience with thermal-sprayed silver oxide–containing hydroxyapatite coating implant. J Arthroplasty. 2016;31:1498–503.
Kabata T, Maeda T, Kajino Y, Hasegawa K, Inoue D, Yamamoto T, Takagi T, Ohmori T, Tsuchiya H. Iodine-supported hip implants: short term clinical results. BioMed Res Int. 2015;2015: 368124.
Zagra L, Gallazzi E, Romanò D, Scarponi S, Romanò C. Two-stage cementless hip revision for peri-prosthetic infection with an antibacterial hydrogel coating: results of a comparative series. Int Orthop. 2019;43:111–5.
Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–69.
Sugano N, Hamada H, Uemura K, Takashima K, Nakahara I. Numerical analysis evaluation of artificial joints. J Artif Organs in press.
Ma R, Tang T. Current strategies to improve the bioactivity of PEEK. Int J Mol Sci. 2014;15:5426–45.
Lee JH, Jang HL, Lee KM, Baek HR, Jin K, Hong KS, Noh JH, Lee HK. In vitro and in vivo evaluation of the bioactivity of hydroxyapatite-coated polyetheretherketone biocomposites created by cold spray technology. Acta Biomater. 2013;9:6177–87.
Wang H, Xu M, Zhang W, Kwok DT, Jiang J, Wu Z, Chu PK. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials. 2010;31:8181–7.
Nakahara I, Takao M, Goto T, Ohtsuki C, Hibino S, Sugano N. Interfacial shear strength of bioactive-coated carbon fiber reinforced polyetheretherketone after in vivo implantation. J Orthop Res. 2012;30:1618–25.
Boschetto F, Marin E, Ohgitani E, Adachi T, Zanocco M, Horiguchi S, Zhu W, McEntire BJ, Mazda O, Bal BS, Pezzotti G. Surface functionalization of PEEK with silicon nitride. Biomed Mater. 2020;16:015015.
Ishikawa M, de Mesy Bentley KL, McEntire BJ, Bal BS, Schwarz EM, Xie C. Surface topography of silicon nitride affects antimicrobial and osseointegrative properties of tibial implants in a murine model. J Biomed Mater Res A. 2017;105:3413–21.
Pezzotti G, Marin E, Adachi T, Lerussi F, Rondinella A, Boschetto F, Zhu W, Kitajima T, Inada K, McEntire BJ, Bock RM, Bal BS, Mazda O. Incorporating Si3N4 into PEEK to produce antibacterial, osteocondutive, and radiolucent spinal implants. Macromol Biosci. 2018;18: e1800033.
Omichi M, Matsusaki M, Kato S, Maruyama I, Akashi M. Enhancement of the blood compatibility of dialyzer membranes by the physical adsorption of human thrombomodulin (ART-123). J Biomed Mater Res B Appl Biomater. 2010;95:291–7.
Griffiths JT, Roumeliotis L, Elson DW, Borton ZM, Cheung S, Stranks GJ. Long term performance of an uncemented, Proximally hydroxyapatite coated, double tapered, titanium-alloy femoral stem: results from 1465 Hips at 10 years minimum follow-up. J Arthroplasty. 2021;36:616–22.
Antoci V, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin Orthop Relat Res. 2007;462:200–6.
Philp AM, Raja S, Philp A, Newton Ede MP, Jones SW. The effect of vancomycin and gentamicin antibiotics on human osteoblast proliferation, metabolic function, and bone mineralization. Spine. 2017;42:202–7.
Kuźmińska A, Butruk-Raszeja BA, Stefanowska A, Ciach T. Polyvinylpyrrolidone (PVP) hydrogel coating for cylindrical polyurethane scaffolds. Colloids Surf B Biointerfaces. 2020;192: 111066.
Acknowledgements
We would like to thank Professor Yasuhiko Tabata of Kyoto University for his advice on this research project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Enami, H., Nakahara, I., Ando, W. et al. Osteocompatibility of Si3N4-coated carbon fiber-reinforced polyetheretherketone (CFRP) and hydroxyapatite-coated CFRP with antibiotics and antithrombotic drugs. J Artif Organs 26, 144–150 (2023). https://doi.org/10.1007/s10047-022-01340-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-022-01340-5