Abstract
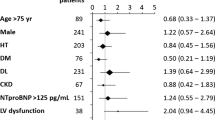
Preoperative cardiopulmonary exercise testing (CPET) is well validated for prognostication before advanced surgical heart failure therapies, but its role in prognostication after LVAD surgery has never been studied. VE/VCO2 slope is an important component of CPET which has direct pathophysiologic links to right ventricular (RV) performance. We hypothesized that VE/VCO2 slope would prognosticate RV dysfunction after LVAD. All CPET studies from a single institution were collected between September 2009 and February 2019. Patients who ultimately underwent LVAD implantation were selectively analyzed. Peak VO2 and VE/VCO2 slope were measured for all patients. We evaluated their association with hemodynamic, echocardiographic and clinical markers of RV dysfunction as well mortality. Patients were stratified into those with a ventilatory class of III or greater. (VE/VCO2 slope of ≥ 36, n = 43) and those with a VE/VCO2 slope < 36 (n = 27). We compared the mortality between the 2 groups, as well as the hemodynamic, echocardiographic and clinical markers of RV dysfunction. 570 patients underwent CPET testing. 145 patients were ultimately referred to the advanced heart failure program and 70 patients later received LVAD implantation. Patients with VE/VCO2 slope of ≥ 36 had higher mortality (30.2% vs. 7.4%, p = 0.02) than patients with VE/VCO2 slope < 36 (n = 27). They also had a higher incidence of clinically important RVF (Acute severe 9.3% vs. 0%, Severe 32.6% vs 25.9%, p = 0.03). Patients with a VE/VCO2 slope ≥ 36 had a higher CVP than those with a lower VE/VCO2 slope (11.2 ± 6.1 vs. 6.0 ± 4.8 mmHg, p = 0.007), and were more likely to have a RA/PCWP ≥ 0.63 (65% vs. 19%, p = 0.008) and a PAPI ≤ 2 (57% vs. 13%, p = 0.008). In contrast, peak VO2 < 12 ml/kg/min was not associated with postoperative RV dysfunction or mortality. Elevated preoperative VE/VCO2 slope is a predictor of postoperative mortality, and is associated with postoperative clinical and hemodynamic markers of impaired RV performance.







Similar content being viewed by others
References
Kormos RL, Cowger J, Pagani FD, et al. The society of thoracic surgeons intermacs database annual report: evolving indications, outcomes, and scientific partnerships. Ann ThoracSurg. 2019;107:341–53.
Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–6.
Grant AD, Smedira NG, Starling RC, Marwick TH. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am CollCardiol. 2012;60:521–8.
LaRue SJ, Raymer DS, Pierce BR, Nassif ME, Sparrow CT, Vader JM. Clinical outcomes associated with INTERMACS-defined right heart failure after left ventricular assist device implantation. J Heart Lung Transplant. 2017;36:475–7.
STS INTERMACS Database. Appendix A—adverse event definitions. UAB School of Medicine.
Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J ThoracCardiovascSurg. 2010;139:1316–24.
Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am CollCardiol. 2008;51:2163–72.
Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2010;105:1030–5.
Fitzpatrick JR, Frederick JR, Hsu VM, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant. 2008;27:1286–92.
Atluri P, Goldstone AB, Fairman AS, et al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann ThoracSurg. 2013;96:857–63.
Soliman OII, Akin S, Muslem R, et al. Derivation and validation of a novel right-sided heart failure model after implantation of continuous flow left ventricular assist devices: The EUROMACS (European Registry for Patients with Mechanical Circulatory Support) right-sided heart failure risk score. Circulation. 2018;137:891–906.
Kukucka M, Stepanenko A, Potapov E, et al. Right-to-left ventricular end-diastolic diameter ratio and prediction of right ventricular failure with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2011;30:64–9.
Wang Y, Simon MA, Bonde P, et al. Decision tree for adjuvant right ventricular support in patients receiving a left ventricular assist device. J Heart Lung Transplant. 2012;31:140–9.
Loghmanpour NA, Kormos RL, Kanwar MK, Teuteberg JJ, Murali S, Antaki JF. A Bayesian model to predict right ventricular failure following left ventricular assist device therapy. JACC Heart Fail. 2016;4:711–21.
Loforte A, Montalto A, Musumeci F, et al. Calculation of the ALMA risk of right ventricular failure after left ventricular assist device implantation. ASAIO J. 2018;64:e140–7.
Kalogeropoulos AP, Kelkar A, Weinberger JF, et al. Validation of clinical scores for right ventricular failure prediction after implantation of continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2015;34:1595–603.
Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–86.
Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016;4:607–16.
Arena R, Myers J, Abella J, et al. Defining the optimal prognostic window for cardiopulmonary exercise testing in patients with heart failure. Circ Heart Fail. 2010;3:405–11.
Arena R, Myers J, Abella J, et al. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115:2410–7.
Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail. 2008;1:227–33.
Methvin AB, Owens AT, Emmi AG, et al. Ventilatory inefficiency reflects right ventricular dysfunction in systolic heart failure. Chest. 2011;139:617–25.
STS INTERMACS Database. Appendix A—adverse event definitions. https://www.uab.edu/medicine/intermacs/intermacs-documents: UAB School of Medicine
Whipp BJ, Ward SA, Wasserman K. Ventilatory responses to exercise and their control in man. Am Rev Respir Dis. 1984;129:S17-20.
Pinsky MR. The right ventricle: interaction with the pulmonary circulation. Crit Care. 2016. https://doi.org/10.1186/s13054-016-1440-0.
Benton CR, Sayer G, Nair AP, et al. Left ventricular assist devices improve functional class without normalizing peak oxygen consumption. ASAIO J. 2015;61:237–43.
Dunlay SM, Allison TG, Pereira NL. Changes in cardiopulmonary exercise testing parameters following continuous flow left ventricular assist device implantation and heart transplantation. J Card Fail. 2014;20:548–54.
Kukucka M, Potapov E, Stepanenko A, et al. Acute impact of left ventricular unloading by left ventricular assist device on the right ventricle geometry and function: effect of nitric oxide inhalation. J ThoracCardiovascSurg. 2011;141:1009–14.
Meineri M, Van Rensburg AE, Vegas A. Right ventricular failure after LVAD implantation: prevention and treatment. Best Pract Res ClinAnaesthesiol. 2012;26:217–29.
Tang PC, Haft JW, Romano MA, et al. Right ventricular failure following left ventricular assist device implantation is associated with a preoperative pro-inflammatory response. J CardiothoracSurg. 2019;14:80.
Braghieri L, Mondellini GM, Javaid A, et al. Upfront RVAD strategy and early clinical outcomes in LVAD patients. J Heart Lung Transplant. 2020;39:S25.
Acknowledgements
Dr. Grinstein is a consultant for Medtronic and speakers bureau for Abbott. Dr. Sheikh receives institutional research support from Abbott. Dr. Najjar receives research support and is a consultant for Abbott. All other authors have no relevant disclosures. No funding was received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Grinstein—Consultant for Medtronic and Speakers Bureau for Abbott; Sawalha, Medvedofsky, Ahmad, Hofmeyer, Rodrigo, Kadakkal, Barnett, Kalantari, Talati, Zaghol and Molina—none; Sheikh—institutional research support from Abbott; Najjar—research support and consultant for Abbott.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grinstein, J., Sawalha, Y., Medvedofsky, D.A. et al. VE/VCO2 slope predicts RV dysfunction and mortality after left ventricular assist device: a fresh look at cardiopulmonary stress testing for prognostication. J Artif Organs 24, 425–432 (2021). https://doi.org/10.1007/s10047-021-01261-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-021-01261-9