Abstract
Purposes
This study explored the association between the nutritional status and survival outcomes after pancreatic cancer surgery and reconsidered surgical indications in octogenarians.
Methods
Three hundred and ninety-three consecutive pancreatic cancer patients who underwent resection were analyzed and grouped according to age (< 70 years old; septuagenarians [70–79 years old], and octogenarians [80–89 years old]). The Charlson age comorbidity index and nutritional parameters were recorded. Survival outcomes and their association with nutritional parameters and prognostic factors were examined.
Results
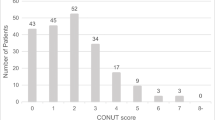
The overall survival was worse in the octogenarians than in other patients. The median overall survivals in the < 70 years old group, septuagenarians, and octogenarians were 27.2, 26.4, and 15.3 months, respectively (P = 0.0828). DUPAN-2 ≥ 150 U/mL, borderline resectable/unresectable tumors, blood loss volume ≥ 500 mL, and blood transfusion were predictors of the overall survival among octogenarians. Nutritional parameter values were worse in the octogenarians than in other patients. The octogenarian age group was not an independent predictor of postoperative complications in a univariate analysis.
Conclusions
Survival outcomes were poor in octogenarians. However, an age ≥ 80 years old alone should not be considered a contraindication for pancreatic cancer surgery. The maintenance of perioperative nutritional status is an important factor associated with the survival.



Similar content being viewed by others
References
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Dias-Santos D, Ferrone CR, Zheng H, Lillemoe KD, Fernandez-Del CC. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Surgery. 2015;157(5):881–7.
Asano T, Yamada S, Fujii T, Yabusaki N, Nakayama G, Sugimoto H, et al. The Charlson age comorbidity index predicts prognosis in patients with resected pancreatic cancer. Int J Surg. 2017;39:169–75.
Bagni K, Chen IM, Johansen AZ, Dehlendorff C, Jensen BV, Hansen CP, et al. Prognostic impact of Charlson’s Age-Comorbidity Index and other risk factors in patients with pancreatic cancer. Eur J Cancer Care (Engl). 2020;29(3): e13219.
Yamada S, Fujii T, Yabusaki N, Murotani K, Iwata N, Kanda M, et al. Clinical implication of inflammation-based prognostic score in pancreatic cancer: Glasgow Prognostic Score is the most reliable parameter. Medicine (Baltimore). 2016;95(18): e3582.
Kato Y, Yamada S, Suenaga M, Takami H, Niwa Y, Hayashi M, et al. Impact of the controlling nutritional status score on the prognosis after curative resection of pancreatic ductal adenocarcinoma. Pancreas. 2018;47(7):823–9.
Nakagawa N, Yamada S, Sonohara F, Takami H, Hayashi M, Kanda M, et al. Clinical implications of naples prognostic score in patients with resected pancreatic cancer. Ann Surg Oncol. 2019;27:887–95.
Network NCC. NCCN clinical practice guidelines in oncology (NCCN Guidelines®). Version 2 2022 ed: NCCN; 2022.
Nakao A, Takagi H. Isolated pancreatectomy for pancreatic head carcinoma using catheter bypass of the portal vein. Hepatogastroenterology. 1993;40(5):426–9.
Japan Pancres Society. Classification of pancretic carcinoma. Fourth English. Tokyo: Kanehara & Co. Ltd; 2017.
McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22(8):881–6.
Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–5.
Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14.
Liaw FY, Huang CF, Chen WL, Wu LW, Peng TC, Chang YW, et al. Higher platelet-to-lymphocyte ratio increased the risk of sarcopenia in the community-dwelling older adults. Sci Rep. 2017;7(1):16609.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–91.
Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg. 2007;205(6):729–34.
Crane CH, Varadhachary GR, Yordy JS, Staerkel GA, Javle MM, Safran H, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29(22):3037–43.
Kinoshita S, Sho M, Yanagimoto H, Satoi S, Akahori T, Nagai M, et al. Potential role of surgical resection for pancreatic cancer in the very elderly. Pancreatology. 2015;15(3):240–6.
Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, Uesaka K. Impact of patient age on the postoperative survival in pancreatic head cancer. Ann Surg Oncol. 2017;24(11):3220–8.
Khan S, Sclabas G, Lombardo KR, Sarr MG, Nagorney D, Kendrick ML, et al. Pancreatoduodenectomy for ductal adenocarcinoma in the very elderly; is it safe and justified? J Gastrointest Surg. 2010;14(11):1826–31.
Melis M, Marcon F, Masi A, Pinna A, Sarpel U, Miller G, et al. The safety of a pancreaticoduodenectomy in patients older than 80 years: risk vs benefits. HPB (Oxford). 2012;14(9):583–8.
Turrini O, Paye F, Bachellier P, Sauvanet A, Sa Cunha A, Le Treut YP, et al. Pancreatectomy for adenocarcinoma in elderly patients: postoperative outcomes and long term results: a study of the French Surgical Association. Eur J Surg Oncol. 2013;39(2):171–8.
Kondo N, Uemura K, Nakagawa N, Okada K, Seo S, Takahashi S, et al. Reappraisal of the validity of surgery for patients with pancreatic cancer aged 80 years or older stratified by resectability status. J Hepatobiliary Pancreat Sci. 2020;27(2):64–74.
Sho M, Murakami Y, Kawai M, Motoi F, Satoi S, Matsumoto I, et al. Prognosis after surgical treatment for pancreatic cancer in patients aged 80 years or older: a multicenter study. J Hepatobiliary Pancreat Sci. 2016;23(3):188–97.
Acknowledgements
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no commercial affiliations that constitute conflicts of interest related to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamada, S., Oshima, K., Nomoto, K. et al. The survival in octogenarians undergoing surgery for pancreatic cancer and its association with the nutritional status. Surg Today (2023). https://doi.org/10.1007/s00595-023-02782-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00595-023-02782-x