Abstract
Background
We aimed to develop criteria for treatment intensification in patients with (1) luminal Crohn’s disease (CD), (2) CD with perianal disease and/or fistula, (3) CD with small bowel stenosis, (4) in the postoperative setting, and (5) for discontinuing or reducing the dose of treatment in patients with CD.
Methods
PubMed and Embase were searched for studies published since 1998 which may be relevant to the five defined topics. Results were assessed for relevant studies, with preference given to data from randomized, controlled studies. For each question, a core panel of 12 gastroenterologists defined the treatment target and developed statements, based on the literature, current guidelines, and relevant additional studies. The evidence supporting each statement was graded using the Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). A modified Delphi process was used to refine statements and gain agreement from 54 Japanese specialists at in-person and online meetings conducted between October 2020 and April 2021.
Results
Seventeen statements were developed for treatment intensification in luminal CD (targeting endoscopic remission), six statements for treatment intensification in perianal/fistulizing CD (targeting healing of perianal lesions and complete closure of the fistula), six statements for treatment intensification in CD with small bowel stenosis (targeting resolution of obstructive symptoms), seven statements for treatment intensification after surgery (targeting endoscopic remission), and five statements for discontinuing or reducing the dose of treatment in patients with CD.
Conclusions
These statements provide guidance on how and when to intensify or de-intensify treatment for a broad spectrum of patients with CD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various guidelines exist on the management of Crohn’s disease (CD), with most advocating a ‘treat-to-target’ (T2T) approach to medical management [1,2,3,4,5,6,7,8,9]. The International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) initiated the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) project to define these targets [2, 10]. The major features of the recent STRIDE-II recommendations are the definition of stepwise targets (specifically short-, intermediate-, and long-term treatment targets) in a T2T approach aimed at improving the long-term quality of life of patients with inflammatory bowel disease; and the use of biomarkers to confirm intermediate targets during T2T.
STRIDE-II offers a practical strategy with optimized objective monitoring by biomarkers and imaging modalities including endoscopy. However, whether we can apply it to all phenotypes of CD, including complicated disease, is an issue for further study.
While the STRIDE targets for CD are important for defining the desired outcome of treatment, they do not provide specific information about when to escalate therapy in the pursuit of that outcome. Such information is important for physicians in clinical practice who must make clinical decisions based not only on treatment goals, but on the clinical, physical, psychosocial, economic, and emotional status of each patient they manage. The TReatment escAlation and DE-escalation decisions in Crohn’s disease (TRADE) group was convened to help answer questions about the criteria for treatment intensification in patients with luminal CD, CD with perianal/fistulizing disease or small bowel stenosis, and in the postoperative setting, and the criteria for discontinuing or reducing the dose of treatment (i.e., de-escalation) in patients with CD. The TRADE group used a modified Delphi consensus process to development statements relating to each question.
Methods
A core group of 12 Japanese gastroenterology specialists (comprising two co-chairs and ten members) was convened to oversee the development of consensus recommendations to answer the following five questions:
-
1.
What are the criteria for treatment intensification in patients with luminal CD?
-
2.
What are the criteria for treatment intensification in patients with CD with perianal or fistulizing disease?
-
3.
What are the criteria for treatment intensification in patients with CD with small bowel stenosis?
-
4.
What considerations are essential for treatment intensification in patients with CD in the postoperative setting?
-
5.
What are the considerations for de-escalation (i.e., discontinuing or reducing the dose of treatment) in patients with CD?
In addition to the core group, an additional 42 gastroenterologists (Supplementary Table 1) were selected for the Delphi panel based on their expertise and specialist knowledge. These 54 individuals comprised the TRADE group. All members of the TRADE group specialized in the management of inflammatory bowel disease (IBD) [as surgeons, endoscope specialists, and physicians], worked at tertiary hospitals, medical centers or clinics in Japan, and were familiar with Japanese clinical practice guidelines relating to IBD.
For each question, a literature search of Embase and Medline was conducted in December 2019. The first four searches used key words related to CD and treatment intensification or escalation, and the searches for questions 2, 3 and 4 included key words related to perianal/anal involvement, stenosis, and surgery, respectively. The fifth search used key words related to CD and treatment de-escalation or discontinuation. All searches were limited to studies in human subjects published in English. The search period was the end of 1998 to December 2019 for searches 2, 3 and 4, and the end of 2014 to December 2019 for searches 1 and 5, due to the large volume of data published on these latter topics. Complete details of the literature search and results are shown in the Supplementary Table 2.
The search results were assessed by the core group for relevant studies based on the title and abstract, with preference given to data from randomized, controlled studies. For each question, the treatment target was defined and statements were developed, based on the literature results, current guidelines, and relevant additional studies known to the authors. The evidence supporting each statement was graded using the Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009) [11].
Four online meetings of the TRADE group, including the 12 core members, were held between 06 June and 07 August, 2020 to develop the statements. Each meeting related to one question (question 1) or two questions, with questions 2 and 4 covered in two separate meetings. At each meeting, two individuals were elected as section leaders at each meeting (with the exception of the meeting for question 1, for which there were four leaders) who were responsible for drafting the statements relevant to each question. The corresponding authors of this paper attended all meetings. Consensus for each statement was subsequently assessed at a meeting on 03 October 2020. Fifty-one of the 54 gastroenterologists in the TRADE group (eleven individuals from the core group and 40 member gastroenterologists) attended the meeting in October 2020 and acted as a survey panel. This meeting consisted of two parts: during the first part, panel members discussed each statement in detail and made amendments where necessary; in the second part, the revised statements were presented for voting. In the latter part of the meeting, the panel used a web-based digital survey system to express their agreement or disagreement with each statement, using a 9-point scale (1, least agree to 9, most agree, with scores 7, 8, 9 defined as ‘agree’ for the purposes of consensus). The results of the voting were used to determine the level of consensus for each statement. Two rounds of voting were held, with consensus defined by a specified percentage of respondents scoring the statements as 7, 8, or 9. In the first round, a consensus of ≥ 75% led to inclusion of the statement and < 75% led to the second round of voting, which used the same score inclusion policy. The voting and consensus rates are shown in Supplementary Table 3. A consensus was reached for all statements. Final statements and the rationale for each statement were circulated to 54 members for final approval on 30 April 2021.
Consensus statements arising from each question are presented below followed by a discussion supporting these recommendations.
Consensus recommendations
Question 1. What are the criteria for treatment intensification in patients with luminal CD?
Target: endoscopic remission.
Statement 1.1: Initial identification of primary non-response or loss of response after initial improvement is based on signs and symptoms, biomarkers and global assessment of the patient’s condition, including extraintestinal manifestations, by the treating physician (Evidence level 1a) | Voting agreement rate 41/46 (89.1%) |
Non-response to medical therapy, whether primary or after an initial remission/improvement, is the most commonly cited reason for treatment escalation in the literature [6, 12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Real-world evidence also suggests that dose escalation is associated with shorter disease duration and corticosteroid use and occurs at a lower frequency in clinical practice than in trial settings [48]. Suboptimal response is usually based on signs and symptoms of CD (such as weight loss, diarrhea, abdominal pain), extraintestinal manifestations (e.g., iritis or arthritis), use of symptomatic treatments (e.g., for diarrhea or pain), blood test parameters (e.g., systemic markers of inflammation such as C-reactive protein [CRP]), and patient general well-being. While some research has been undertaken to develop a clinical risk score that will help to identify patients who would benefit from treatment escalation [49], there is currently no accepted and validated scoring system for this purpose that is recommended in clinical guidelines.
Statement 1.2: The presence of extraintestinal manifestations is often a sign of ongoing intestinal inflammation and may indicate a need for more intensive therapy (Evidence level 2b) | Voting agreement rate 42/44 (95.5%) |
Up to 50% of patients with CD develop extraintestinal manifestations of the disease; the most common types being arthropathy, dermatological conditions (e.g., erythema nodosum, pyoderma gangrenosum, Sweet’s syndrome), oral aphthous ulcers, ocular conditions (e.g., episcleritis or uveitis) and hepatobiliary conditions (most commonly primary sclerosing cholangitis) [50]. These conditions may indicate intestinal inflammation, but can also occur independently (Supplementary Table 4) [50].
Given the potential relationship between extraintestinal manifestations and bowel inflammation, the presence of extraintestinal manifestations may prompt more intensive anti-inflammatory therapy [51]. Patients with extraintestinal manifestations may also benefit from referral to another specialist (e.g., dermatologist, ophthalmologist, rheumatologist) and local and/or symptomatic therapies [51].
Statement 1.3: The Crohn’s Disease Activity Index (CDAI) or Harvey–Bradshaw Index (HBI) can be used to measure clinical disease activity, with both measures being equally effective. Because of its simplicity, the HBI is recommended as the instrument of choice for assessing clinical disease activity in routine clinical practice; an HBI score of > 4 is indicative of active disease (Evidence level 1b, 5) | Voting agreement rate 42/44 (95.5%) |
Clinical studies in patients with CD often use the Crohn’s Disease Activity Index (CDAI in adults or Pediatric CDAI [PCDAI] in children) to assess signs and symptoms (Supplementary Table 5) [52]. A CDAI score of < 150 is used to define remission [5, 53]. A recent literature survey in Japan found that the CDAI was the most commonly used index in clinical trials [54]. However, the threshold for treatment escalation based on CDAI score differs between studies. Several randomized controlled trials published since 2014 used the CDAI as part of the criteria to define escalation [14, 55,56,57,58]. In their 2008 study comparing a top-down vs a step-up approach to CD treatment, D’Haens and colleagues used an increase in CDAI score of ≥ 50 points to escalate treatment [57]. The GAIN study used a CDAI score of 220–450 in patients already taking infliximab to identify those who may benefit from a change in anti-TNFα therapy [58]. In the CALM study, the CDAI score used to define moderate to severe CD in the patient inclusion criteria was 220–450 in patients not receiving prednisone at baseline, 200–450 in patients receiving prednisone at a dose of ≤ 20 mg for ≥ 7 days before baseline, and > 150 to 450 in patients receiving prednisone at a dose of > 20 mg for ≥ 7 days before baseline [14]. The TAILORIX study used a hierarchical approach to treatment escalation criteria; the first step in this hierarchy was a CDAI score > 220 and the second step used a CDAI score of 150–220 for two consecutive weeks [55]. The only recent randomized trial in children used a PCDAI score of ≥ 10 to define loss of response [12]. In all of these studies, CDAI criteria alone were insufficient to consider treatment escalation (see further discussion below) [12, 14, 55, 56].
The two predominant symptoms measured in the CDAI (stool frequency and abdominal pain) are individually only weakly correlated with endoscopic findings, but the correlation tends to be a little stronger for stool frequency (r = 0.36) than for abdominal pain (r = 0.22) [59]. Presumably, this is because pain is complex and has many potential causes including some unrelated to active inflammation (e.g., disrupted motility, fibrosis, adhesions, strictures) [59].
While the CDAI is widely used in a clinical research context, it may not be practical for use in clinical practice, because it is complicated to calculate, varies between patients (especially in relation to items such as abdominal pain) and relies on patients completing symptom diaries, uses a poorly defined standard for body weight, does not accurately assess the severity of fistulas and stenosis, and is weighted heavily toward diarrheal symptoms [1, 54]. The simpler Harvey–Bradshaw index (HBI) may be a more suitable tool for the assessment of disease activity in clinical practice (Supplementary Table 6), but this is also weighted by diarrhea meaning that CD patients with an increased stool frequency are likely to show a score disproportionate to disease activity [1, 54]. Studies using the HBI generally define lack of response as a score of ≥ 5 [28, 42], although some have used a score of > 4 [60, 61]. HBI scores are highly correlated with CDAI scores (correlation coefficient of 0.8–0.93) [61, 62]; an HBI score > 4 is equivalent to a CDAI score of > 150 [61].
Statement 1.4: While disease signs and symptoms are often the first indicator that treatment is suboptimal, treatment escalation should not be undertaken without confirming the presence of ongoing intestinal inflammation (Evidence level 3, 5) | Voting agreement rate 44/44 (100%) |
In clinical practice, physicians generally do not use a formal tool to assess disease activity, but rather assess their patient’s condition based on physical examination, patient report, and blood test results. This is a valid approach, but decisions on treatment escalation should not be solely based on signs of disease activity, without confirming the presence of ongoing intestinal inflammation. Neither clinical signs of disease activity nor patient reports of worsening symptoms correlate strongly with ongoing inflammation in CD [1, 63, 64]. Some CD patients have concomitant functional bowel conditions that can worsen symptoms in the absence of ongoing inflammation [65]. Conversely, some patients in clinical remission may have mucosal inflammation [65], as demonstrated in the ACCENT 1 study [66]. The STRIDE II guidelines for treatment outcomes highlight the importance of endoscopic healing as an intermediate-to-long-term treatment goal, alongside the need to resolve/minimize symptoms in the short term [10]. A recent retrospective study of CD patients found that, despite a moderate level of agreement with endoscopic healing, histologic healing was more closely associated with decreased risk of clinical relapse, medication escalation, or corticosteroid use [67]. As noted in a recent review, histological remission is increasingly becoming a novel target within the concept of disease clearance comprising clinical, endoscopic and microscopic remission [68]. However, the extent to which this more rigorous treatment goal can be adopted in daily clinical practice requires further examination.
Statement 1.5: Patients with worsening signs or symptoms of CD during treatment should undergo assessment for intestinal inflammation, using biomarkers, small bowel radiography, endoscopy or cross-sectional imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) (Evidence level 5) | Voting agreement rate 45/45 (100%) |
Statement 1.6: Endoscopy is the established standard for the assessment of intestinal inflammation (Evidence level 5) | 46/47 (97.9%) |
Statement 1.7: It is important to visualize the small bowel mucosa, using balloon-assisted endoscopy or capsule endoscopy, when active small bowel disease is suspected (Evidence level 2a) | 47/47 (100%) |
Statement 1.8: When endoscopy or capsule endoscopy is not feasible or practical, noninvasive imaging using magnetic resonance enterography (MRE), CT enterography (CTE) or ultrasound is the preferred modality (Evidence level 5) | 47/47 (100%) |
Statement 1.9: CT use should be minimized in patients with CD to limit lifetime exposure to radiation (Evidence level 1a/1b) | 47/47 (100%) |
For objective assessment of intestinal inflammation, UK guidelines and STRIDE I/II recommendations recommend endoscopy as the preferred modality [1, 2, 10]. In Japan, the most common type of CD is ileocolonic [69, 70], so ileocolonoscopy is the most widely used modality. However, many Japanese patients (between 16% and 36%) do not have colonic involvement [69, 70]. Ileocolonoscopy does not reach portions of the bowel proximal to the terminal ileum. Therefore, in patients with known small bowel disease or upper gastrointestinal symptoms (e.g., anemia, weight loss, cramping after meals, nausea/vomiting, upper abdominal pain), other modalities that visualize the small bowel may be needed. For these patients, physicians can use small bowel radiography, capsule endoscopy or balloon-assisted endoscopy to assess the small bowel [1, 71,72,73,74].
Capsule endoscopy is a valid, noninvasive alternative to conventional endoscopy, and has a sensitivity of 83% and specificity of 53% for the diagnosis of active CD in the small bowel [75]. However, there are currently no validated criteria for the interpretation of capsule endoscopy and there is a small risk of retention, particularly in patients with strictures [1]. A few studies identified in our search used capsule endoscopy to determine treatment decisions [76,77,78,79,80]. In one prospective observational study, patients aged ≥ 10 years with CD and a Lewis score of > 135 on capsule endoscopy could have their treatment escalated, irrespective of symptoms [76]. Of 40 patients, 29 (72.5%) patients underwent treatment escalation and experienced an improvement in CDAI and Lewis score. While these data are promising, the study was small, not randomized, and there was no control group to assess outcomes in patients with a high Lewis score who did not undergo treatment escalation. Another observational study found that panenteric capsule endoscopy resulted in treatment intensification in 38.7% of patients overall but especially among those with an established diagnosis whose disease was active (64.6% of patients) [80]. A Japanese study analyzed the correlations between several biomarkers and the Capsule Endoscopy Crohn's Disease Activity Index (CECDAI) or Lewis score [77]. This found that capsule endoscopy could identify intestinal abnormalities in most CD patients in clinical remission with detection rates using CECDAI (81.0%) and the Lewis score (85.7%) being highly correlated. Finally, a retrospective single-center study from Japan found that patients with a Lewis score > 270 (and Prognostic Nutritional Index < 45) were at increased risk of exacerbation, prompting the need for treatment escalation at these thresholds [78]. Among pediatric patients, a new score (Capsule endoscopy–Crohn’s disease index, CE–CD) reliably and simply predicted the need for treatment escalation in this specific population [79].
However, the extent of intestinal inflammation can also be assessed using cross-sectional imaging with magnetic resonance enterography (MRE), computed tomography enterography (CTE) or ultrasound (US) [73]. These techniques have the advantage of being able to image parts of the bowel that are inaccessible by endoscopy, such as the small bowel. Another advantage of cross-sectional techniques is that they provide an assessment of transmural disease, such as wall thickening and mural edema, and can identify other abdominal or pelvic complications, such as mesenteric venous thrombosis/occlusion, sacroiliitis, pancreatitis, cholelithiasis, or kidney stones [81,82,83,84]. A recent analysis of US performance in predicting the need for treatment intensification, which included 89 CD patients, found that mean bowel wall thickness and the presence of the bowel wall Doppler signal were independent predictors of the need for treatment escalation [85].
CTE has a sensitivity of 67% and specificity of 100% for the diagnosis of active CD in the small bowel. Guidelines agree that the use of CT imaging should be minimized because of the long-term risks associated with cumulative radiation exposure [1, 4, 5, 65, 83]. Some authors have suggested that CTE is best suited to elderly patients because of the risk of radiation exposure, and the requirement for fewer breath holds (compared with MRE) [81].
Research suggests that CTE, MRE and small bowel follow-through (SBFT) are similarly accurate for the diagnosis of active CD in the small bowel, but both CTE and MRE show better interobserver agreement than SBFT, and are better at detecting extraintestinal complications [84]. Consensus recommendations from the Society of Abdominal Radiology (SAR) suggest when each type of enterography may be best utilized (Supplementary Table 7) [83]. These SAR guidelines also describe the features of CD on MRE or CTE imaging and how these features should be reported by radiologists to gastroenterologists [83], but they do not make a specific recommendation that one type of imaging is preferable to another or that any particular scoring system should be used. A list of advantages and disadvantages of endoscopic and imaging techniques are shown in Table 1 [72,73,74, 81, 82, 86,87,88,89,90,91].
Treatment escalation based on close endoscopic monitoring (> 1 endoscopy within 26 weeks) is associated with an increased probability of mucosal healing (compared with less frequent endoscopic monitoring) [13]. UK guidelines note that the choice of monitoring modality (inflammatory biomarkers, endoscopy, imaging) and the interval between assessments should be individualized based on the site, distribution and severity of the patient’s CD, and patient preference [1]. For example, disease in the upper gastrointestinal tract or proximal small bowel is a predictor of relapse, while inflammation in the terminal ileum is a risk factor for stricturing/penetrating disease (see Sect. 3). Similarly, disease in the colon is a risk factor for perianal disease, which is a poor prognostic indicator in itself (see Sect. 2) [92].
Statement 1.10: MRE is as accurate as CTE for assessing the severity of intestinal inflammation in patients with CD (Evidence level 1a) | Voting agreement rate 46/47 (97.9%) |
A meta-analysis of data from studies using cross-sectional imaging indicates that CTE and MRE are highly accurate for grading CD severity, but US is less so (Supplementary Table 8) [93]. Contrast-enhanced US (CEUS) has greater diagnostic accuracy than standard US (90–95% for CEUS vs 69–88% for wall thickness and/or color Doppler flow on US) [94].
MRE and CTE appear to have similar sensitivity and specificity for detecting active inflammation [1], including in the small bowel [75, 84], although a comparative UK study suggests that MRE may have greater specificity in the assessment of small bowel disease (extent and severity).
Statement 1.11: Both the CDEIS and the SES-CD are reliable. SES-CD is the recommended scoring system for endoscopic evaluation of intestinal inflammation, based on its validity and simplicity (Evidence level 2a) | Voting agreement rate 46/47 (97.9%) |
The most common scoring systems for endoscopic activity in CD are the Crohn’s disease Endoscopic Index of Severity (CDEIS; Supplementary Table 9) [95], and Simplified Endoscopic activity Score for Crohn’s disease (SES-CD; Supplementary Table 10) [96]. The CDEIS produces a score of between 0 and 44, while the SES-CD produces a score of between 0 and 56 [97].
The CDEIS is widely used in clinical trials, but is complex to calculate and requires training/experience to accurately estimate the extent of diseased mucosal surfaces and distinguish between superficial and deep lesions [81]. The SES-CD was developed to overcome these limitations and develop a scoring system more suited to everyday clinical practice [81]. SES-CD scores are highly correlated with CDEIS scores (r = 0.938; p < 0.0001) [98]; both scores are highly reproducible and show good inter-observer consistency [97, 98]. However, in contrast to the CDEIS, the SES-CD does not take into account the number of explored segments and characterizes ulcers by size rather than by depth [97].
Both the CDEIS and the SES-CD are reliable and validated instruments for quantifying the severity of mucosal inflammation [97]. SES-CD shows slightly better correlation with histopathological scores of disease severity compared with the CDEIS (SES-CD: r = 0.600 and CDEIS: r = 0.554). However, as described earlier, there is poor correlation between clinical indices of disease activity, such as the CDAI and endoscopic indices; r = 0.254 for CDEIS and CDAI, r = 0.267 for CDEIS and HBI, r = 0.235 for SES-CD and CDAI, and r = 0.204 for SES-CD and HBI [99]. Based on the simplicity/ease of use of the SES-CD and the better correlation with histopathology, SES-CD is recommended as the endoscopic index of choice in the current statements.
To date, no threshold levels for either scoring system have been defined as a measure of response or non-response [81, 97, 100]. Our search identified several studies that used the CDEIS and SES-CD indices for the assessment of mucosal inflammation published since 2014, but none used them to make treatment escalation decisions. In all studies, the indices were used for assessing treatment outcomes after escalation [14, 26, 36, 41, 55]. The Organization for the Study of Inflammatory Bowel Disease (IOIBD) used a Delphi process to define such thresholds, and ranked the following as their top 4 definitions of endoscopic remission [97]:
-
1.
SES-CD of 0 to 2
-
2.
CDEIS < 6 (endoscopic remission)/CDEIS < 3 (complete endoscopic remission)
-
3.
Absence of ulceration
-
4.
CDEIS score of ≤ 4.
In STRIDE II, the target for endoscopic remission in CD have been proposed as follows:
-
1.
SES-CD (ulcer subscores = 0 (including aphthous ulcerations) < 3
-
2.
CDEIS (no ulcers) < 3.
Other authors have defined thresholds for the severity of mucosal inflammation (Supplementary Table 11), but these thresholds were arbitrarily set [99]. What is not clear from the literature is whether the absence of remission (based on these scores) should be an indication for treatment escalation. The European Crohn’s and Colitis Organisation (ECCO) guidelines suggest that SES-CD > 6 is indicative of need to escalate treatment to biologic therapy [9]. Based on the data and these recommendations, it would appear that an SES-CD score > 6 during treatment indicates moderate to severe CD which requires treatment escalation.
Histologic assessment of a biopsy sample is not needed to make decisions about treatment escalation. A 2017 Cochrane review noted that there was no uniform methodology for biopsy collection (site) or handling (fixation, sectioning), and none of the available histologic scoring indices has been fully validated [101]. Similarly, STRIDE guidelines (both I and II) note that histologic remission is an important adjunctive assessment measure but should not be a treatment target, because there is insufficient evidence about its clinical value [2, 10].
Histologic healing as a therapeutic endpoint in CD remains challenging. To date, no consensus has been reached to define histologic remission, and none of the 14 different numerical indices been fully validated in all four operating characteristics of reliability, validity, responsiveness and feasibility.
Statement 1.12: Symptomatic patients: Most patients with worsening signs or symptoms of CD plus objective markers of inflammation (elevated serum level of CRP or leucine-rich α2 glycoprotein (LRG) and/or fecal calprotectin [FC] level) are candidates for treatment escalation, before confirmation of intestinal inflammation by endoscopy or imaging. Patients with fever or abdominal pain should undergo imaging studies to rule out abscess formation. (Evidence level 1a) | Voting agreement rate 37/45 (82.2%) |
The STRIDE II guidelines noted “serum and fecal biomarkers are endorsed as intermediate medium-term feasible treatment goals, meaning that at times treatment could be revisited solely based on these tests, to facilitate care in the clinic setting. Elevated serum or fecal biomarkers at times may suffice to revise treatment and at other times require endoscopic confirmation to document the extent and severity of the disease prior to major treatment changes” [10]. Therefore, treatment intensification can be considered in patients with high CRP or FC values and confirmation with endoscopy may also be necessary. However, final endoscopic confirmation is usually needed to assess the achievement of the treatment target of endoscopic remission. The exception to this recommendation is the patient with fever, who should be assessed for the presence of intestinal abscess.
Levels of CRP, FC, LRG and stool lactoferrin (SL) are useful noninvasive markers of inflammation, although FC appears a stronger predictor of relapse in patients with ulcerative colitis than in patients with CD [102, 103]. CRP is a nonspecific marker of inflammation, whereas FC and SL reflect leukocyte trafficking in the gut and are specific to intestinal inflammation [104]. A meta-analysis of studies defined the diagnostic accuracy of these biomarkers for endoscopically active disease (Table 2) [104]. Both CRP levels and FC levels significantly correlate with endoscopic scores of inflammation [105]. LRG levels correlate significantly with the CDAI score in Japanese patients with CD (r = 0.361; p = 0.044), whereas CRP levels do not (r = 0.150; p = 0.405) [103], and the correlation between CDAI score and FC is of borderline significance in Japanese patients with CD (r = 0.283; p = 0.0565) [106]. The sensitivity of FC is greater than that of CRP for detecting mild mucosal inflammation, while CRP appears to be a better biomarker of inflammation in patients with severe systemic inflammation [107]. Typically, in the literature, the threshold CRP level is ≥ 5 mg/L and FC level is > 250 μg/g when these biomarkers are used to define criteria for treatment escalation in CD patients [12, 14, 23, 33, 43, 55, 108]. An SL level of 7.25 μg/g is defined as the optimal threshold for diagnosing endoscopically active disease [104].
Statement 1.13: Asymptomatic patients: Those with elevated levels of CRP or FC should be considered for assessment of intestinal inflammation using endoscopic or imaging studies (Evidence level 1a/1b) | Voting agreement rate 45/45 (100%) |
The CALM study (2018) was a randomized comparison of two treatment escalation strategies: one in which treatment escalation decisions were based on CDAI scores ± prednisone use (the clinical management group) and the other in which treatment escalation was based on CDAI scores, elevated levels of CRP (≥ 5 mg/L) and FC (≥ 250 μg/g), and prednisone use [14]. Treatment escalation steps were undertaken at 12-week intervals in patients who met the defined criteria in each group and consisted of (1) starting adalimumab administered every 2 weeks, (2) then weekly, (3) then adding azathioprine. De-escalation could be undertaken at weeks 24 and 36 in patients who did not meet the criteria for treatment failure. Significantly more patients in the tight-control group met the primary endpoint of mucosal healing on endoscopy at week 48 compared with patients whose treatment was escalated in response only to disease activity scores [14]. Other endpoints (including deep remission) also occurred at a significantly higher rate in the tight-control group than the clinical management group. A recently published post hoc analysis of CALM showed that FC < 250 μg/g is useful for predicting endoscopic mucosal healing in CD patients [109].
A systematic review of treat-to-target approaches supports the results of the CALM study, i.e., that treatment escalation based on biomarker evidence of inflammation and signs/symptoms of disease activity is associated with better outcomes than escalation based on signs/symptoms alone [13]. However, more recent individual studies continue to provide conflicting perspectives. For example, a recent Japanese study of 46 CD patients followed up for 1 year following diminished infliximab effects after therapy intensification found that outcomes were improved in patients who received infliximab treatment intensification based on endoscopic findings of exacerbations to a greater extent than in patients monitored via clinical symptoms [110]. On the other hand, the recent STARDUST trial compared a treat-to-target strategy involving early endoscopy, regular biomarker and clinical symptom monitoring, with a clinical maintenance strategy in CD patients with moderate-to-severe disease receiving ustekinumab [111]. Results showed that, at week 48, endoscopic response, endoscopic remission, mucosal healing, and clinical remission were not significantly different when comparing the two approaches, underlying a need for ongoing assessment of studies in this area.
Other biomarkers are in development, including serum calprotectin (SC) [25]. SC may overcome some of the limitations of FC (day-to-day variability, effect of storage conditions, low positive predictive value), but has not yet demonstrated a correlation with mucosal healing [25], and has not been used to determine treatment escalation in any study we could identify.
It is also possible that patients without symptoms have ongoing inflammation, so elevated CRP and FC levels in patients without symptoms should prompt an assessment of mucosal inflammation with endoscopy or other imaging modality [2]. Similarly, there is a possibility for active small bowel lesions in asymptomatic patients with normal CRP or FC levels [112].
Statement 1.14: The choice of monitoring modality (biomarkers, endoscopy, imaging) and the interval between assessments should be individualized based on the site, distribution and severity of the CD, patient preference, local availability of imaging exams/expertise, and cost (Evidence level 5) | Voting agreement rate 45/45 (100%) |
As described earlier (see Supplementary Table 7 and Table 1), each method for assessing intestinal inflammation has advantages and disadvantages. As a result, major guidelines recommend that the choice of modality is individualized based on the site, distribution and severity of the patient’s CD, patient preference, local availability of imaging machines, local expertise, and cost [1]. We recommend monitoring biomarker levels every 3 months in patients with CD, even when the disease is clinically inactive.
Statement 1.15: Measurement of anti-tumor necrosis factor (TNF)-α drug levels and/or antibody titers may be useful to determine the need for switching or intensifying biological therapy in patients with primary or secondary non-response, but is not necessary at routine check-ups in patients in remission (Evidence level 1b, 5) | Voting agreement rate 46/46 (100%) |
Pharmacokinetic or pharmacodynamic causes may underlie poor primary or secondary response to treatment with anti-TNFα therapy [16, 113]. There is considerable evidence to support checking trough drug levels in patients with persistent or worsening symptoms during anti-TNFα therapy [12, 22, 40, 43, 45, 55, 56, 114,115,116,117,118,119,120,121]. In patients with a primary non-response in the first 4–6 weeks, low trough levels of anti-TNFα agents are unlikely to be related to anti-biologic antibody formation [16, 113]. Some commentators have suggested that patients with a primary non-response after 14 weeks of treatment should have antibody titers measured to determine the best approach [16]. US and UK guidelines recommend that patients with a loss of response after initial symptomatic improvement should have drug levels and antibody levels measured [1, 122], but ECCO guidelines do not recommend this approach; because the evidence to support it is weak [9]. The presence of low drug levels and absent or low antibody titers may be an indication to increase the dose of the biological or shorten the dosing interval [113]. Indeed, an analysis of the DIAMOND study comparing adalimumab with adalimumab plus azathioprine in immunosuppressant-naive CD patients found a significant difference in trough levels of adalimumab in patients with and without clinical remission [123]. Further analysis suggested the significance of therapeutic drug monitoring including both trough levels and 6-thioguanune nucleotide levels when managing CD patients receiving combination therapy and independent associations between female sex, increased body and low adalimumab trough levels. If there is evidence of a high antibody titer, or if drug levels are high and antibody titers are absent or low, then a switch to a different biologic agent may be indicated [113]. Using antibody titers and drug levels to guide treatment decisions, in addition to symptoms, does not necessarily improve outcomes compared with using symptoms alone to guide treatment intensification [55, 56], but it does reduce costs [40, 56].
There is some evidence from randomized controlled trials and retrospective observations of real-world clinical practice to suggest that therapeutic drug monitoring (e.g., measuring anti-TNFα blood levels at each maintenance infusion) can improve outcomes or delay the need for surgery in adults and children with CD [12, 43, 124]. However, there are no currently agreed target thresholds for trough anti-TNFα levels and the comprehensive UK treatment guidelines do not advocate therapeutic drug monitoring in the absence of signs/symptoms of active disease [1]. Currently available evidence/expert opinion favors the measurement of drug levels in a reactive manner, i.e., when there are signs of increased disease activity, rather than in a proactive manner, i.e., at each routine check-up [125, 126]. However, when patients have sub-therapeutic drug levels in association with a low titer of anti-drug antibody, optimizing immunomodulator therapy or increasing the dose of anti-TNFα therapy may help to avoid future relapse [125].
Statement 1.16: Asian patients with CD should be tested for the NUDT15 p.Arg139Cys genotype before initiating thiopurine treatment (Evidence level 4–5) | Voting agreement rate 45/46 (97.8%) |
Statement 1.17: When thiopurine therapy is insufficient, consider optimization by increasing the dose (Evidence level 4–5) | 41/41 (100%) |
Asian patients require lower doses of thiopurines to achieve therapeutically effective blood levels than Caucasians do, and are more susceptible to certain adverse events, such as alopecia and leukopenia [127]. Thiopurine metabolite blood levels are determined by metabolic enzymes, which are influenced by pharmacogenetic factors [127]. Low levels of particular thiopurine metabolites usually reflect genetic variants, which can help determine whether a patient may benefit from a dose increase, whereas a therapeutic/normal level in a non-responsive patient is an indication to switch treatment [1]. The major metabolites affected by genetic variants are the 6-thioguanine nucleotides (6-TGN) [127].
In Caucasian individuals, variations in thiopurine metabolism are mainly due to polymorphisms in the gene for thiopurine S-methyl transferase (TMPT) [1, 127]. However, this is not the case in Japanese patients who show low TMPT activity and a very low prevalence of TMPT mutations [127, 128]. In Japan and other east Asian countries, variants in the gene for nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) are a common reason for variation in response and tolerability of thiopurines [127, 129]. NUDT15 variants reduce the conversion of active thiopurine metabolites to inactive metabolites, leading to higher levels of active metabolites and an increased risk of developing adverse events. Currently, the p.Arg139Cys genotype of NUDT15 is probably the most relevant pharmacogenetic test for Japanese patients with CD [129]. Approximately 1.1% of the Japanese population has the Cys/Cys genotype of p.Arg139Cys [127]. Based on the profile of enzymes affected by NUDT15 mutations, the most relevant metabolite to measure in Japanese patients on thiopurines is DNA-incorporated thioguanine (DNA-TG) [127]. However, a recent study Japanese study found that a simple high-resolution melt analysis technique correctly identified the main NUDT15 genotypes in 1236 of 1241 cases [130].
Another less important mutation that affects thiopurine metabolism in Asian individuals occurs in the ITPA gene (specifically a 94C>A single nucleotide polymorphism), which appears to predict the development of adverse effects [127]. The metabolites affected by ITPA variants are the TGN, the same metabolites affected by TMPT mutations in Caucasians [127].
Question 2. What are the criteria for treatment intensification in patients with CD with perianal or fistulizing disease?
Target for perianal lesions: healing.
Target for fistula: complete closure.
Statement 2.1: In patients with CD, perianal disease is associated with numerous poor outcomes and a greater risk of disease relapse prompting a lower threshold for treatment intensification (Evidence level 2c, 5) | Voting agreement rate 45/45 (100%) |
Perianal disease is associated with numerous poor outcomes, including high risks of complicated disease course, suboptimal response to therapy or relapse, need for surgery and stoma creation, and hospitalization [131,132,133,134,135,136]. Diagnostically, perianal lesions may be the first presentation of CD and patients presenting with perianal lesions suspected to be related to CD require evaluation of the bowel. Colonic disease location and penetrating behavior appear associated with the development of perianal disease [131, 136]. In particular, perianal disease appears to be most strongly associated with rectal involvement, having previously been noted in approximately 90% of such patients [137]. In patients with active perianal disease, numerous factors, including complex fistulizing disease, a history of abscess, antibiotic treatment, colonic involvement, smoking, and stricturing phenotype, have been identified that predict poor treatment response [134]. Discontinuation of anti-TNFα therapy in patients who had previously experienced benefit from anti-TNFα therapy, and stricturing phenotype have been associated with a greater risk of relapse in patients with perianal fistulas treated with infliximab or adalimumab [138]. Furthermore, perianal disease is associated with significant impairment in quality of life, especially related to physical symptoms and fecal incontinence [139, 140]. Consequently, perianal disease represents a prompt for intensification of treatment reflected by the fact that the proportion of patients who receive immunomodulator therapy and/or anti-TNFα agents has been shown to increase after the diagnosis of perianal disease [135, 136].
Statement 2.2: Perianal CD is best managed by a multidisciplinary team including surgeons, coloproctologists, and specialist gastroenterologists (Evidence level 5) | Voting agreement rate 46/47 (97.9%) |
Because fistula management is complex and perianal lesions can be the first presentation of CD, treatment decisions should be made in a multidisciplinary context with input from specialist gastroenterologists, surgeons, coloproctologists, and other medical staff [1, 141]. Consideration should also be given to the patient’s potential sense of embarrassment in relation to their symptoms and the impact of complex perianal fistulas on different aspects of life, including relationships, social life and work/professional life.
Statement 2.3: Initial assessment of the type, extent, and severity of perianal disease as well as the determination of treatment goals is crucial to guiding treatment strategy, both in general and in relation to treatment intensification (Evidence level 5) | Voting agreement rate 46/46 (100%) |
Perianal disease may include fistulizing lesions (fistulas and abscesses) and non-fistulizing lesions (ulceration, fissures, and strictures/stenosis) [142, 143]. Various classification systems have been developed to describe perianal disease both in terms of pathological type, anatomical extent, and severity.
The Parks classification of perianal fistulas is well-known but is not specific to perianal CD or suitable for the routine clinical management of perianal CD [144]. Rather, a practical way to categorize fistulas is as simple and complex. Simple fistulas are superficial, or low inter- or trans-sphincteric fistulas with a single internal opening and no purulent reservoirs, rectovaginal fistula, or anorectal stricture [131]. Complex fistulas occur at higher levels, with more than one opening, and often in association with abscesses, anal stenosis or other disease (Supplementary Fig. 1, Table 3).
Because fistulotomy is associated with nonhealing wounds and a subsequent high rate of proctectomy or incontinence in patients with CD, the American Gastroenterological Association Technical Review on Perianal CD issued in 2003 has recommended that fistulotomy is relatively contraindicated for simple fistula with rectal inflammation or for complex fistula [145]. Rather, it should be indicated only for simple fistula with a single external opening without rectal inflammation.
A previous classification for perianal CD (the Cardiff classification) grades ulceration, fistulas, and strictures on a severity scale of 0 to 2 and classifies fistulas as low or high (Supplementary Table 12) [145].
Malignancy, such as rectal or anal carcinoma, should be excluded at the earliest point of assessment. Determining the extent and presence of fistula openings, their course and the presence of fluid or pus collections is critical when planning treatment [131]. Ulceration, when present, may consist of superficial fissures or cavitating ulcers involving the anal canal or lower rectum with possible extension to perianal skin (aggressive ulceration). Strictures are usually irreversible, leading to anal stenosis or extra-rectal stricture, with severe impact but may in some cases be reversible when physically expanded. The presence of any ulceration and/or stricture in the rectum (proctitis) or inflammation and/or stricture of the anal canal is an important component for fistula assessment [146]. A perianal abscess is clinically defined as fluctuation and radiologically defined as a confined fluid collection and is important to detect during assessment as their timely management minimizes the risk of further septic complications [146].
Fistula activity should be assessed using grading scales, such as the Perianal Disease Activity Index (PDAI) [147], which assesses both disease severity and response to therapy based on measures of quality of life (pain/restriction of activities, and restriction of social activities) and disease severity (fistula discharge, type of perianal disease, and degree of induration). The original description of the PDAI details restriction of sexual activity, although restriction of social activity may be used when these scales are collected insufficiently.
Statement 2.4: Treatment goals should be determined according to the condition of individual patients with the primary aim of complete closure and, if not possible or expected, the secondary aim of symptomatic relief (Evidence level 5) | Voting agreement rate 46/46 (100%) |
Treatment goals for each patient should be based on an assessment of the type, extent, and severity of perianal disease as previously described. In general, the primary aim for perianal fistulas is complete fistula closure. Reported fistula closure rates with various therapies are described in more detail in the discussion of statement 2.6. However, studies reveal that complete closure is frequently not achievable in all patients even with combined medical and surgical therapy. In such patients, it is recommended to pursue the secondary aims of symptomatic improvement or resolution, improvement in quality of life, and prevention of septic complications. Ultimately, the long-term goal of treatment is to maintain anal function.
In terms of achieving treatment goals, history and local physical examination is well-suited to monitoring discharge, local symptoms, and restrictions on physical and sexual or social activity. On the other hand, objective imaging studies can more easily assess the location, distribution, activity, abscess formation, type of fistula and factors that may be contributing to symptoms and signs (e.g., collections, stricturing). Consensus guidelines from France have outlined targets to be attained, such that treatment intensification is not required, in the domains of symptoms, physical examination findings, and MRI findings [148].
Statement 2.5: The sequence and choice of treatment strategy, including surgical procedures and use of anti-TNFα therapy, should be based on stepwise assessment of the lesion(s) and response to treatment (Evidence level 1, 5) | Voting agreement rate 45/45 (100%) |
In patients with active fistulizing perianal disease, the choice of treatment strategy should be based on a stepwise assessment of the lesion(s) and their response to treatment as outlined in Fig. 1.
Patients with suspected coexisting abscesses should be evaluated early by surgical consultation and examination under anesthesia (EUA) for possible drainage and/or seton insertion. Antibiotics, especially metronidazole and ciprofloxacin, are commonly used and recommended for initial symptom control. Although antibiotics alone may reduce fistula drainage and achieve fistula closure in some patients, this response may not always be sustained after cessation [144, 149]. Fistula closure rates of 20–50% have been noted with metronidazole but recurrence rates of up to 80% have also been noted following cessation [149]. As a result, antibiotics have been viewed as a bridge to more definitive treatment strategies [144]. Imaging studies, including proctoscopy, proctosigmoidoscopy, and pelvic MRI are strongly recommended to delineate the anatomy of the fistula tract(s) and determine if complicated or uncomplicated fistulizing disease is present. CT may also be used, where other options are not readily available, although the diagnostic accuracy of CT is lower than that of MRI. However, initial assessment should not be restricted to pelvic MRI or CT but also include evaluation for the presence of colon and rectal lesions. Patients with complicated disease are generally referred initially for surgical consultation, whereas those with uncomplicated disease can be initially treated with medical therapy. For the closure of perianal fistulas, both infliximab and adalimumab have been shown to be effective in a number of studies [138, 150,151,152,153,154,155], and are recognized as the standard of treatment to induce symptomatic response and complete closure [144, 146]. Consensus statements also suggest that anti-TNFα therapy is combined with immunomodulator therapy (thiopurines), again based on low-quality evidence, to increase the chances of fistula closure and long-term remission (see statement 2.6 for study details) [144]. Optimization of anti-TNFα therapy by measurement of trough concentrations and assessment of anti-drug antibodies may improve outcomes in patients with perianal disease. Observational studies have shown that higher trough levels of anti-TNFα therapy are associated with more favorable fistula response and lower rates of relapse in patients with perianal fistula [156,157,158,159]. Although trough measurement is not indicated in Japan, consideration should be given to increasing the trough concentration by increasing the dose of anti-TNFα therapy or shortening the administration period in cases of insufficient therapeutic response.
The extent of fistula closure should be assessed throughout these steps to guide treatment decisions. Recent guidelines have defined response and remission to treatment in patients with fistulizing, perianal CD as follows [144]:
-
Symptomatic response: Meaningful improvement in symptoms of pain and discharge as judged by both the patient and physician in the absence of remission. As noted previously, symptomatic response should not be considered the primary aim of treatment. However, symptomatic response is useful to assess early treatment response and may be a secondary aim if the primary aim of closure is not achievable. The degree of symptomatic response can be determined by reference to the PDAI (Supplementary Table 13). Reduction in the total score by ≥ 4 points suggests meaningful recovery, whereas a reduction of ≤ 3 points suggests no change or possible exacerbation of disease [145].
-
Symptomatic remission: This is marked by absence of both pain and discharge from the fistula tract. In terms of the PDAI scoring system, remission would be characterized by a score of 0 in the items related to discharge, pain and restriction of activities, and possibly restriction of sexual activity.
-
Radiographic response: Severity of MRI findings can be quantified using scoring systems, such as the van Assche score, which provides specific subscores in the domains: number of fistula tracks, location, extension, hyperintensity on T2-weighted images, collections (> 3 mm diameter) and rectal wall involvement [160]. Van Assche scores > 15 have been associated with significantly lower long-term healing rates in a prospective, observational study of 70 patients with CD and anal fistulas [161]. A similar but more recently developed scoring index (MAGNIFI-CD) based on 6 items also determines perianal fistulizing activity but with improved operating characteristics compared with previous systems [162]. Accurate assessment of the number, location and extent of tracts, especially if multiple, may be difficult. However, hyperintensity on T2-weighted images, presence of collections and rectal wall involvement in terms of thickening are more readily assessable.
-
Radiographic remission: In conjunction with use of the radiographic scoring systems described above, true radiographic remission can be viewed simply as the absence of inflammation in any fistula tract and the absence of any abscess.
-
Complete remission: This can be viewed as the combined presence of symptomatic and radiographic remission.
Response and remission can be determined by fistula drainage assessment such as that used in clinical trials, which defined improvement as a decrease from baseline of ≥ 50% in the number of open draining fistula for at least two consecutive visits (≥ 4 weeks) and remission as closure of all fistula that were draining at baseline for at least two consecutive visits (≥ 4 weeks) [163]. However, such strict definitions may be difficult to apply in routine practice, where the close observation periods of clinical trials may not be practically feasible. In such patients, clinicians should also consider predictors of poor outcomes in patients with perianal fistulizing disease, including complex fistulas, colonic location, stricturing phenotype, and a history of perianal abscess [134, 138, 164].
Statement 2.6: Treatment intensification in patients with perianal disease should be guided by the nature of the lesion(s) and response to initial therapy (Evidence level 1, 5) | Voting agreement rate 36/37 (97.3%) |
Treatment intensification in patients with perianal disease should be based on the treat to target principle with the aim of optimizing therapy to achieve fistula closure according to physical examination findings and objective diagnostic imaging studies. However, treatment intensification may not achieve this aim, in which case secondary goals, such as those in relation to symptomatic and functional improvement, should be set.
Assessment of actual and likely response is crucial when considering treatment intensification. Prognostic factors for greater chance of successful healing include the absence of proctitis, short duration of fistulizing disease, nonsmoking status, and simple fistula [165]. Complex fistulas, on the other hand, predict a worse response [164, 166]. As during the initial phase of treatment, inadequate symptomatic response or loss of symptomatic response should prompt consideration of repeated surgical management to clarify the need for surgery before intensifying treatment. If surgery is deemed necessary, surgery should be performed first before simply intensifying medical treatment. However, fistula relapse is common following perianal surgery [167], and evidence from at least one retrospective review suggests that drug escalation after initial surgery is associated with a significantly reduced likelihood of reoperation [168]. In patients treated with anti-TNFα therapy who have achieved a symptomatic response, ongoing reassessment is needed to determine if treatment can be maintained at the same level or if the dose of anti-TNFα agent should be increased. Immunomodulator therapy may also be required if this has not already been commenced. In patients who have undergone treatment optimization, ongoing monitoring based on physical examination findings and diagnostic imaging studies should be maintained with the primary aim of fistula closure.
Favorable response to both anti-TNFα therapy and thiopurines, including in terms of closure, have been noted in the literature. A meta-analysis of 5 randomized trials reported a higher rate of fistula response with azathioprine or 6-mercaptopurine than placebo (odds ratio, 3.09 [95% CI 2.45–3.91]) [169]. Complete fistula response (i.e., closure) was higher with 6-mercaptopurine (31%) than placebo (6%) in an early long-term randomized controlled trial of 83 patients [170]. Fistula closure with infliximab 5 mg/kg was also significantly higher than with placebo (55% vs 13%; p = 0.001) in a randomized, double-blind trial with a median duration of response of approximately 3 months [153]. The ACCENT II trial confirmed the long-term response of infliximab with a sustained response (in terms of predefined reduction in draining fistulas) noted in twice as many patients treated with infliximab than placebo (46% vs 23%; p = 0.001) [155]. Similar results were seen in the CHARM trial, which demonstrated a significantly higher complete fistula closure rate for adalimumab than for placebo after 26 weeks (30% vs 13%; p < 0.001) [171].
A practical question in terms of treatment intensification concerns timing of dose increase and dose combination. In the ACCENT II trial involving infliximab monotherapy [155], the peak time to loss of response among patients with a response at randomization was approximately 10 weeks. Hence, a period of 3–6 months seems reasonable to assess initial response to therapy and decide whether further increase in anti-TNFα dose or combination with thiopurine treatment may be necessary. There are limited studies to thoroughly assess the efficacy of anti-TNFα therapy and thiopurines in combination and results appear to be mixed. The rate of fistula response during the open-label phase of the ACCENT II study was identical in patients receiving concomitant immunomodulator therapy and those receiving infliximab monotherapy alone [155]. However, an open-label case series suggested that combination therapy with infliximab and an immunosuppressant (purine analog or methotrexate) was associated with a greater likelihood of first perianal fistula closure (HR 2.58, 95% CI 1.16–5.60) compared with infliximab alone, especially in patients who were naïve to immunosuppressants [172]. These results are supported by those of a prospective observational cohort study of 41 patients with perianal fistula which found that combination anti-TNFα and thiopurine therapy led to clinical benefit (remission or response) in 58% of patients up to the end of the 3-year follow-up period [173]. Despite issues with the evidence for combination therapy, many consensus guidelines recommend the use of a thiopurine in combination with anti-TNFα therapy, including at initiation of therapy.
Treatment intensification strategies are more complex in cases of stenosis. Treatment pathways for anorectal stenosis associated with CD depend on the type of stenosis (fibrous, inflammatory) or presence of dysplasia, which generally necessitates proctectomy regardless of the grade [142, 174]. First-line treatment of an inflammatory anal or rectal stenosis is medical treatment with failure of treatment prompting optimization of therapy or dilatation, which is the first-line strategy for isolated fibrous stenosis [174]. Medical therapy with an anti-TNFα agent and immunomodulator may also be used in combination with surgical drainage and dilatation in patients with concomitant stenosis and anoperineal suppuration [142, 174]. Finally, the possibility of malignancy should be considered throughout the clinical course, especially when there are symptoms such as worsening pain and increased drainage, or when the treatment effect is poor.
Question 3. What are the criteria for treatment intensification in patients with CD with small bowel stenosis?
Target: Resolution of obstructive symptoms.
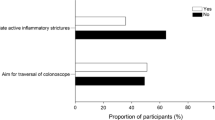
Statement 3.1: Assessment of small bowel stenosis is indicated in CD patients with new onset or worsening of obstructive symptoms, including acute abdominal distension, cramping, nausea, vomiting, and abdominal pain (Evidence level 5) | Voting agreement rate 45/46 (97.8%) |
Stenosis is a common complication of CD, particularly in patients with small bowel disease [175], or after surgery [176]. The definition of this condition varies between studies [177], but it is generally accepted to be a narrowing of the bowel lumen caused by thickening of all layers of the bowel wall [177, 178]. Narrowing of the bowel can relate to edema associated with acute inflammation, whereas fibrosing stenosis develops after prolonged inflammation [179]. Therefore, any narrowing can be predominantly inflamed, predominantly fibromatous or mixed [180]. Fibrosis occurs because of the pleiotropic effects of inflammatory mediators [177]. Chronic inflammation stimulates fibroblast activation in the extracellular matrix, leading to collagen deposition, tissue remodeling and stenosis formation [179]. The term stricture is often used to describe fibrotic narrowing, with or without an inflammatory component [83].
Because CD tends to be progressive, the current hypothesis is that the bowel wall remodelling occurs after years of accumulating inflammatory damage, resulting in stenotic complications [177]. However, stenosis may sometimes be present at diagnosis [181]. Therefore, physicians should have a high index of suspicion for small bowel stenosis in patients who present with or develop obstructive symptoms, particularly if they have ileal CD.
The key symptoms of small bowel stenosis are acute abdominal distension, cramping, nausea, vomiting, abdominal pain, which is typically postprandial [182]. Patients with such signs and symptoms should be investigated for the presence of stenosis [1]. Bouhnik and colleagues have developed the CD obstructive score, which incorporates the severity and duration of obstructive pain, the presence and duration of nausea and/or vomiting, whether the patient is voluntarily limiting their diet because of symptoms, and the consequences of obstructive symptoms (e.g., hospitalization) [183]; however, this score is not widely used in clinical practice or in clinical research.
Statement 3.2: A number of imaging modalities can be used to identify small bowel stenosis, including cross sectional imaging modalities (US, CTE and MRE), small bowel radiography and balloon-assisted endoscopy. If available, MRE is the preferred imaging modality for identifying small bowel stenosis (Evidence level 2b, 4) | Voting agreement rate 42/44 (95.5%) |
As described in more detail in statement 1.7, cross-sectional imaging modalities are particularly useful for the assessment of the small bowel [72, 75, 81,82,83,84], and are able to detect mural changes associated with stenosis [175]. Endoscopy is particularly good at identifying inflammation on the luminal surface, but endoscopists may not be able to determine whether the narrowing is fibrotic or edematous (inflammatory) [176, 180]. US has the advantage of being non-invasive and allowing interaction with the patient during the procedure, so the patient can direct the sonographer to the areas of greatest discomfort, which can help to detect lesions responsible for the symptoms [176]. However, diagnostic yield may be lower with ultrasound than with magnetic resonance enteroclysis [180].
A recent comprehensive systematic review of the literature by Bettenworth and colleagues on different radiographic modalities including ultrasound, CT, MRE, and hybrid PET was conducted [175]. The reported sensitivity and specificity of these modalities are summarized in Supplementary Table 14 [175, 179].
Based on an analysis of the literature, these authors recommend MRE as the preferred imaging modality based on its accuracy, availability, and lack of exposure to radiation [175]. This recommendation is supported by data from a Japanese study, which found that MRE has 82.8% accuracy for diagnosis of small bowel stenosis, and 87.8% accuracy for the diagnosis of major small bowel stenosis [184]. However, this study [184] also found that MRE has lower sensitivity for detecting small bowel stenosis (58.8% for major stenosis and 40.8% for any stenosis) than the sensitivity reported in the review by Bettenworth et al. (75–100%) [175]. Balloon-assisted endoscopy, in addition to MRE, may be beneficial if the patient has obstructive symptoms as indicated in statement 3.3 [185]. Unfortunately, in patients with stricturing disease, SES-CD based on conventional ileocolonoscopy does not correlate with MaRIA score nor does it predict the need for treatment escalation, [186]. This is likely because the stenosis prevents deep insertion of the scope for many patients, thereby limiting the ability of endoscopy to assess inflammation. A more recent review of MRE over a 5-year period from Australia confirmed that MRE is useful in patients with strictures and was able to predict the need for surgery as well as which patients may benefit from treatment intensification [187].
Contrast enhancement techniques can help differentiate between fibrotic and edematous narrowing [175, 179], but there are limited comparative data to guide a recommendation for one imaging modality over another in this regard. Some studies have suggested that CT modalities have limited ability to detect fibrosis [188].
Statement 3.3: Even if cross-sectional imaging, including MRE, is negative, balloon-assisted endoscopy is recommended in patients with obstructive symptoms; this allows the option of performing endoscopic balloon dilatation in suitable candidates (Evidence level 2b, 4) | Voting agreement rate 45/46 (97.8%) |
Data from Japan indicate that about one in four CD patients with a negative result on MRE will have stricturing present on balloon-assisted endoscopy [185]. In this study, balloon-assisted endoscopy was able to detect stenosis in MRE-negative patients that were less severe than the ones in MRE-positive patients, i.e., stenosis with greater diameter, shorter length, and with a lower incidence of prestenotic dilatation [185]. Importantly, 10.8% of the patients who were MRE-negative but had positive findings on balloon-assisted endoscopy required further surgery [185]. Therefore, we recommend balloon-assisted endoscopy especially for symptomatic patients and those with a negative result on MRE to ensure detection of stenosis. An additional advantage of balloon-assisted endoscopy is that endoscopic balloon dilatation can be performed if a stenosis is identified and the patient is a suitable candidate (see statement 3.5). A recent study from Japan highlighted the role of balloon-assisted enteroscopy for evaluating deep small bowel lesions even in patients in clinical remission [189]. Multivariate logistic regression analysis found that the Harvey–Bradshaw Index and a partial Simple Endoscopic Score for CD independently predicted relapse. The authors concluded that balloon-assisted enteroscopy is important for ongoing evaluation of small bowel lesions even among patients in remission.
Statement 3.4: Treatment selection in stenotic CD depends on whether the stenosis is primarily inflammatory or fibrotic. Surgical treatment or endoscopic balloon dilatation should be considered for fibrotic-predominant stenosis indicated by clinical features and imaging findings (Evidence level 2a) | Voting agreement rate 39/46 (84.8%) |
Guidelines recognize the importance of distinguishing between predominantly inflammatory and predominantly fibrotic stenosis, when determining treatment approaches in patients with small bowel stenosis [1]. Optimized medical therapy is indicated when the stenosis is predominantly inflammatory, whereas patients with fibrotic stenosis are better managed by surgery or endoscopic balloon dilatation (Fig. 2) [1].
However, differentiating between a predominantly inflammatory and predominantly fibrotic stenosis requires careful consideration of a range of factors including inflammatory biomarkers (such as CRP levels), clinical and imaging features, and response to medical therapy.
While CRP levels can indicate the presence of a spike in inflammatory activity, there is currently no biomarker that can determine which patients have primarily inflammatory vs fibrotic stenosis. The observational CREOLE study identified the following clinical and imaging factors as significantly associated with treatment success (and, therefore, a primarily inflammatory stenosis): severe obstructive symptoms lasting < 5 weeks, moderate prestenotic dilatation (diameter of 18–29 mm) and marked delayed phase enhancement on MRE [183]. The study authors proposed a stenosis scoring system and suggested that the effectiveness of adalimumab varies according to the score. While the CREOLE study was not randomized, it provides some guidance on the patients who are most likely to benefit from a trial of adalimumab. No comparable data were available to determine whether the same criteria could be used to identify suitable patients for treatment with other anti-TNFα therapy.
Obstructive symptoms that do not resolve with intensified medical therapy suggest a fibrotic etiology; patients with such ongoing symptoms should be assessed for a more interventional approach, such as endoscopic balloon dilatation or surgery.
Patients without signs of inflammatory stenosis (defined above) probably have fibrotic stenosis and are unlikely to respond to medical treatment, so immunosuppressive therapy will subject them to adverse events for minimal clinical benefit [183]. These patients may benefit from endoscopic balloon dilatation, strictureplasty or surgical resection [1, 190].
Statement 3.5: Before endoscopic balloon dilatation therapy, the length, location and angulation of the stenosis should be assessed. Endoscopic balloon dilatation should not be undertaken if fistulas or deep ulcers are present within the stenosis (Evidence level 2a) | Voting agreement rate 46/46 (100%) |
Endoscopic balloon dilatation is indicated for a short stenosis (≤ 5 cm) [191,192,193], that does not respond to medical therapy [178]. Longer, severe, frequently recurring, or more complex stenosis may best be treated with strictureplasty or surgical resection [1, 178, 190].
A recent meta-analysis by Bettenworth and colleagues (2020) examined outcomes of endoscopic balloon dilatation specifically in patients with small bowel stenosis, excluding those with colonic stenosis or stenosis of the ileocolonic anastomosis [194]. Approximately 82% of these patients achieved symptomatic relief after the procedure [194]. Factors significantly associated with increased risk of unsuccessful outcome in patients with small bowel stenosis were endoscopic evidence of active disease in jejunum and/or proximal ileum and Asian race [194]. Irrespective of the site of the stenosis, factors that increase the risk of complications after endoscopic balloon dilatation are a predominantly inflammatory stenosis with a high level of disease activity, multiple stenosis, a tortuous or tethered small bowel or significant stenosis angulation, complete bowel obstruction, adjacent penetrating ulcer or intra-abdominal collection, fistulization within 5 cm of the area to be dilated, or stenosis caused by extrinsic compression (e.g., adhesions) [190, 191]. Contraindications to endoscopic balloon dilatation are stenoses associated with an abscess, phlegmon, fistula, high-grade dysplasia, or malignancy [190].
Two systems are available for endoscopic balloon dilatation: over-the-wire (OTW) balloon catheter and through-the-scope (TTS) balloon but the TTS system tends to be used more frequently [190, 192]. Balloon dilatation can be graded, in which the balloon size is incrementally increased, or non-graded using a single balloon size, but the graded approach appears to be the most common [192], and is predictive of a successful outcome in patients with small bowel stenosis [194]. The balloon catheter can be inserted via an antegrade or retrograde approach. The choice of system, approach (antegrade/retrograde), technique (graded vs non-graded), dilatation time, and size of the balloon should be tailored to each patient according to their anatomical site and features, including luminal diameter and stenosis length [190, 192].
Technical failure (inability to dilate the stenosis) occurs in about 6–11% of patients undergoing endoscopic balloon dilatation of the small bowel [194, 195], and major complications occur in 4–6% [192, 194,195,196].
Statement 3.6: Endoscopic balloon dilatation contributes to the avoidance or delay of surgery (Evidence level 2a) | Voting agreement rate 43/45 (95.6%) |
Most patients who develop a stenosis will experience a recurrence after endoscopic balloon dilatation and need to undergo a second dilatation or surgery [194, 196]. Data from the only prospective study on outcomes after endoscopic balloon dilatation of a small bowel stenosis in Japan showed that 36% of patients required redilatation at 2 years, and 53% needed redilatation at 3 years [197]. This is lower than the proportion estimated in the meta-analysis by Bettenworth and colleagues; that study estimated that 55.4% of patients require re-dilatation within 2 years [194, 196].
However, data on surgery avoidance were similar in the long-term Japanese study and in the meta-analysis. The long-term Japanese study by Hirai and colleagues reported that 21% of patients who had undergone endoscopic balloon dilatation of a small bowel stenosis underwent surgery within 2 years, and 27% required surgery within 3 years [197]. A nationwide retrospective study from Japan reported surgery-free rates of 74.0% at 1 year, 54.4% at 5 years, and 44.3% at 10 years after endoscopic balloon dilatation of small bowel stenosis [198]. The use of immunomodulators or anti-TNFα therapy after the onset of obstructive symptoms significantly increased the likelihood of avoiding surgery after endoscopic balloon dilatation. Other factors that were associated with being able to avoid surgery were non-stricturing or non-penetrating disease at onset, mild symptoms, successful endoscopic balloon dilatation, and stenosis length < 2 cm [198].
Question 4. What considerations are essential for treatment intensification in patients with CD in the postoperative setting?
Target: endoscopic remission.
Statement 4.1: Patients who have undergone surgery for CD are at risk of relapse and reoperation, so postoperative treatment is usually needed. Prior to the scheduled endoscopic assessment after surgery, postoperative treatment decisions should consider preoperative treatment responses and underlying risk factors (i.e., current smoking habit, penetrating disease or previous resection, residual active disease, extensive lesions, perianal disease) (Evidence level 1b, 5) | Voting agreement rate 46/47 (97.9%) |
Approximately 10–38% of patients who undergo surgery for CD have symptoms of clinical recurrence, and 35–93% have endoscopic lesions, within 12 months [199]. Endoscopic disease recurrence predates symptomatic recurrence after surgery for CD [200], and clinical disease activity shows poor correlation with endoscopic disease activity after surgery [201]. Therefore, postoperative management should take a more proactive than reactive course.
Before endoscopic assessment at 6–12 months after surgery, treatment decisions should take into account the patient’s overall risk of recurrence (Supplementary Table 15), response to preoperative treatment, and type of surgery [202].
Currently, there is no validated risk score to guide treatment decisions after surgery [203], and the definition of ‘high risk’ differs in the literature (Supplementary Table 15). The POCER study defined high risk by the presence of current smoking, penetrating disease or previous resection [204], whereas French and UK guidelines, and a recent narrative review, require the presence of ≥ 2 risk factors [1, 6, 202]. Other high-risk clinical or endoscopic features for recurrence include extensive small bowel disease, perianal disease, penetrating disease, endoscopically active disease, and the presence of granulomas or myenteric plexitis [1, 6, 202]. An interval of less than 5 years between a first and second surgery for CD is predictive of the need for a third operation [205].
In the postoperative setting, the use of anti-TNFα therapy may reduce the risk of recurrence in patients with active CD [204, 206], and there is evidence to suggest that these agents are superior to aminosalicylates [203]. Longitudinal data show that the risk of reoperation in CD patients has been significantly lower since 2002, when anti-TNFα therapy was introduced in Japan, although the change may also have been influenced by changes in surgical techniques [206].
The POCER study showed that active care with early endoscopy and treatment escalation based on endoscopic recurrence significantly improves outcomes in CD patients after bowel resection [204]. It also demonstrated that a risk-guided approach to therapy is valuable in the postoperative setting, taking into account the length of the patient’s remaining bowel, and the underlying risk factors for recurrence. Smoking was the most important determinant of disease progression (odds ratio for recurrence of 2.4 [95% CI 1.2–4.8]; p = 0.02) [204]. Therefore, patients should be strongly advised to quit smoking. In patients who are unable to stop smoking after surgical resection, aggressive postoperative treatment of CD with early escalation of medical therapy is warranted.
Statement 4.2: CD patients who have undergone intestinal resection should be endoscopically assessed for intestinal inflammation 6–12 months after surgery, irrespective of their risk profile, and treatment decisions should consider this result. Evidence of mucosal inflammation is required for a diagnosis of CD recurrence, using endoscopy, cross-sectional imaging or biomarker tests (Evidence level 1b, 5) | Voting agreement rate 46/48 (95.8%) |
Based on data from the randomized POCER study [204], most international guidelines agree that, after ileocolonic resection, patients at high risk of recurrence should receive treatment with immunomodulators or anti-TNFα therapy [1, 6, 207]. On the other hand, medical management can be guided by endoscopic recurrence in patients who are not at high risk [202, 208]. We recommend that patients who have undergone ileocolonic resection should undergo endoscopic assessment 6–12 months after surgery, irrespective of their risk profile or medical management. Figure 3 shows the recommended approach to postoperative monitoring and treatment in Japan.
Statement 4.3: Endoscopy is the gold standard for mucosal assessment, but cross-sectional imaging with MRE or US is an alternative if endoscopy is not feasible or appropriate. Balloon-assisted endoscopy or capsule endoscopy are appropriate modalities for small bowel visualization (Evidence level 3a, 5) | Voting agreement rate 47/47 (100%) |
Statement 4.4: The anastomotic site and proximal small bowel should be evaluated after intestinal resection. The presence of a modified Rutgeerts score of i2b or higher after ileocolonic resection is indicative of a high risk of clinical recurrence. Treatment escalation should be considered in patients with active inflammation (e.g., a modified Rutgeerts score ≥ i2b) at any time after bowel resection, regardless of the presence or absence of abdominal symptoms (Evidence level 1b, 3b, 5) | Voting agreement rate 43/46 (93.5%) |
Direct visualization of the bowel via endoscopic techniques is the gold standard for mucosal assessment after surgery [1, 200]. While visualizing the anastomotic site is important, it is also necessary to assess all areas of the bowel likely to be affected by inflammatory changes. The imaging modality should be chosen according to the site of the patient’s CD and the operative procedure, so that the anastomotic site and proximal small bowel can be visualized. If conventional ileocolonoscopy cannot reach the site of anastomosis or the patient has known small bowel involvement, balloon-assisted endoscopy should be considered instead of, or in addition to, ileocolonoscopy. Balloon-assisted endoscopy is usually undertaken via the anal route, but if this technique is unable to reach the anastomosis, endoscopy can be undertaken using the oral route [71]. Capsule endoscopy is a reasonable approach to visualizing the entire bowel in the postoperative setting [209, 210], and is usually performed after successful passage of a patency capsule.
Small bowel enteroclysis/enterography is frequently used for monitoring in the postoperative setting because of their widespread availability in Japan, and ability to accurately detect mucosal abnormalities, although it carries a risk of radiation exposure [211].
Cross-sectional techniques such as bowel US and MRE do not visualize the mucosal surface, and are, therefore, not the preferred method of postoperative assessment, but they can provide complementary information to endoscopy, particularly in relation to involvement of the bowel wall and adjacent tissues [176]. A systematic review and meta-analysis of data with different imaging modalities suggests that they are all highly sensitive in the detection of postoperative recurrence [212], although the sensitivity of MRE for detecting stenosis is only approximately 40% [184]. Contrast enhancement improves the diagnostic accuracy, sensitivity and specificity of bowel US relative to standard bowel US [212, 213], CT or CTE can be used as an option if bowel US or MRE is not available; however, it is important to minimize the risk of radiation exposure.
Patients require regular monitoring after surgery. Patients without evidence of active CD at the first postoperative assessment should undergo regular clinical evaluation, with endoscopy at least every 2 years [208]. The modified Rutgeerts score (Supplementary Table 16) was developed for endoscopic assessment after ileocolonic resection [214]. A score of i2 or higher is a significant predictor of symptomatic recurrence [215]. Currently, there is no accepted scoring system for endoscopic assessment after other types of surgery. In the absence of an accepted scoring system, we recommend using the modified Rutgeerts score after any type of bowel resection.
Statement 4.5: The CD activity index (CDAI) and HBI are poorly indicative of endoscopic recurrence after surgery and are not sensitive enough to monitor early postoperative recurrence (Evidence level 3b) | Voting agreement rate 47/47 (100%) |
Approximately 50% of patients with CD show symptomatic recurrence within 3 years of surgical resection [199]. Therefore, patients require regular monitoring for signs and symptoms of disease activity. However, the CDAI is not sensitive enough to monitor disease activity in the postoperative setting as it shows poor correlation with endoscopic recurrence (Pearson r = 0.07) [216]. As described above, endoscopic monitoring is required after surgery to identify disease recurrence.
Statement 4.6: Biomarkers, including CRP and FC, are useful for adjunct assessment, and FC shows better correlation with endoscopic disease activity after surgery than CRP does (Evidence level 1a) | Voting agreement rate 46/47 (97.9%) |
There is growing evidence for the use of FC in the postoperative monitoring of patients with CD [200], although this test is not yet indicated for postoperative monitoring in Japan. The correlation between FC and disease activity is consistent in patients who have and have not undergone surgery [217]. In the postoperative setting, FC is significantly correlated with endoscopic disease activity (p < 0.05) [201], and has a stronger correlation with disease activity than CRP does [217, 218].
Data from Japan have suggested that serial FC measurements can be used to identify patients who may have postoperative recurrence and would, therefore, benefit from ileocolonoscopy [219]. These authors suggested that the optimal FC threshold for postoperative patients was 140 μg/g [219].
However, this study included only 30 patients, and there is currently not enough evidence to recommend using FC rather than the strategy suggested by the POCER study. In addition, the optimal interval between FC measurements and the most appropriate threshold level of FC has yet to be fully defined [200], and may be affected by the choice of assay [220].
Statement 4.7: In patients with symptoms of CD (pain and/or diarrhea) after surgical resection, other potential causes (such as gastrointestinal infection, bile acid malabsorption or bacterial overgrowth) should be ruled out and recurrent disease confirmed before making a decision on medical therapy (Evidence level 5) | Voting agreement rate 45/47 (95.7%) |
In the early postoperative period, symptoms may indicate intra-abdominal sepsis, anastomotic leak or similar surgical complications [221, 222]. After this time, other potential causes of CD symptoms (particularly diarrhea) may include bile acid malabsorption (after ileocecal surgery), bacterial overgrowth, or enteric infections (e.g., associated with norovirus, Clostridioides difficile, or food-borne pathogens) [1, 221, 223]. In a patient with symptoms but no endoscopic evidence of active disease, other potential causes of gastrointestinal symptoms should be investigated and eliminated before intensifying treatment [1].
Question 5. What are the considerations for de-escalation (i.e., discontinuing or reducing the dose of treatment) in patients with CD?
Statement 5.1: De-escalation of anti-TNFα therapy is associated with an increased risk of relapse, which may be greater in patients receiving anti-TNFα monotherapy (Evidence level 3–4) | Voting agreement rate 46/48 (95.8%) |
As summarized in Supplementary Table 17, relapse rates following discontinuation of anti-TNFα agents reported in clinical studies are high and increase over time. In these studies, a high proportion of patients generally received concomitant immunomodulator therapy (mostly azathioprine), whereas some patients were immunomodulator-naïve and, therefore, were receiving anti-TNFα monotherapy. In studies that examined the influence of concomitant immunomodulator therapy, some found only a small effect of concomitant immunomodulator therapy on the likelihood of relapse. However, a large retrospective, observational study by Casanova found concomitant immunomodulator therapy was significantly protective against relapse (HR 0.67; p = 0.003), which suggests patients who discontinue anti-TNFα monotherapy may be at greater risk of relapse [224].
In STORI, a pivotal, prospective, observational study of 115 patients with CD, relapse rates following discontinuation of infliximab were 44% at 1 year and 52% at 2 years [225]. Other observational studies of biologic therapies have reported 1-year relapse rates of 22–53% following discontinuation, with lower relapse rates typically seen in patients in remission [224, 226,227,228,229,230,231]. Furthermore, greater levels of remission in terms of duration and extent of mucosal healing and other endoscopic evidence of remission also appear to be protective against relapse following treatment de-escalation.
5.2 Discontinuation of immunomodulator monotherapy is associated with an increased risk of relapse (Evidence level 2–4) | Voting agreement rate 45/48 (93.8%) |
CD patients who discontinue immunomodulator monotherapy have been shown to have a high risk of relapse that increases over time (Supplementary Table 18) [232,233,234,235,236,237,238].
Statement 5.3: Discontinuation of immunomodulator therapy may not increase the risk of relapse in patients receiving combination therapy with an anti-TNFα agent and immunomodulator who have achieved steroid-free clinical and biochemical remission for more than 6 months (Evidence level 2, 3, 5) | Voting agreement rate 44/47 (93.6%) |
Concomitant use of immunomodulators with anti-TNFα therapy has been based on the principle of reduction in immunogenicity and improvement in efficacy [239]. Discontinuation of immunomodulator therapy in patients receiving combination treatment with anti-TNFα therapy should be considered separately to that of discontinuation of immunomodulator monotherapy. As shown in Supplementary Table 19 [240,241,242,243], results of different clinical studies are mixed but seem to suggest that immunomodulator discontinuation has less impact among patients on combination therapy than those receiving monotherapy. In particular, in the IMID study of 80 patients with CD, no long-term clinical benefit was observed from continuing immunomodulator therapy beyond 6 months in patients receiving scheduled infliximab, although patients who continued immunomodulator therapy had a lower frequency of infliximab antibodies and higher trough drug concentrations [243]. In support of these findings, a study in Japanese patients with CD also found that continuation of immunomodulator therapy for more than 6 months offered no clear benefit over scheduled anti-TNFα monotherapy in terms of corticosteroid-free clinical remission [240]. However, as with anti-TNFα therapy, the rate of relapse following immunomodulator cessation increases with longer periods of follow-up [244]. This has led to the suggestion that, in patients who receive combination therapy for more than 6 months, triple remission consisting of corticosteroid-free clinical remission, endoscopic remission, and serological remission allows consideration of thiopurine withdrawal [123]. Finally, the impact of immunomodulator discontinuation can be reduced by either partial withdrawal in terms of dose reduction rather than complete cessation or selective withdrawal in patients who have received concomitant treatment for longer periods. In any case, evidence from studies such as DIAMOND2 suggest that an individualized strategy of withdrawal should be implemented [123]. This should include ongoing assessment of measures of efficacy, including endoscopic or cross-sectional imaging parameters, biomarkers, and therapeutic drug monitoring.
Statement 5.4: Key predictors of relapse after de-escalation include younger age, greater disease duration and severity, perianal disease, residual active lesion(s), elevated levels of CRP, FC, and leucocytes, and low hemoglobin levels. (Evidence level 2–4) | Voting agreement rate 45/47 (95.7%) |
Predictors associated with increased relapse risk following discontinuation of anti-TNFα agents and immunomodulators are summarized and compared in Table 4.
Specific additional factors noted in various studies that appear to protect against relapse include: shorter disease duration [245, 246], mucosal healing [247], endoscopic and other evidence of remission [248, 249], optimization or continuation of thiopurine therapy [224, 250], and low or undetectable anti-TNFα levels at discontinuation [246, 247, 251, 252].
Based on these predictors of increased or decreased risk of relapse, patients in deep remission who have clinical, biomarker, and endoscopic factors associated with a lower risk of relapse have the greatest chance of favorable long-term prospects following anti-TNFα therapy discontinuation. However, relatively few patients in a real-world setting fulfil the criteria for withdrawal of biologic treatment even when they meet the lower risk criteria [253], and relatively few patients are able to achieve deep remission, which is the optimal state for considering treatment withdrawal [254].
Before discontinuing or reducing the intensity of any treatment, disease activity should be evaluated from a clinical perspective via a combined approach considering disease history, severity and extent to ensure appropriate patient selection. This approach includes an assessment of the likelihood of relapse based on the identified predictors. Selective patients may have acceptable outcomes with careful de-escalation, especially those who best fit the following profile:
-
Older patients (generally > 22 to 25 years)
-
Short disease duration or a short period between diagnosis and treatment
-
Stable disease, lack of dose escalation or colonic surgery
-
Evidence of remission, including endoscopic findings, mucosal healing and favorable biomarkers (e.g., low CRP, low FC).
In contrast, patients with perianal fistulas have been shown to have a high risk of relapse following cessation of anti-TNFα therapy and discontinuation is generally inadvisable in this specific population [133, 255, 256]. The presence or absence of a history of surgery and disease location have been considered as risk factors of relapse after discontinuation of anti-TNFα therapy but are not consistently reported in the literature. Therefore, more high-quality evidence is needed regarding these as potential risk factors for relapse.
Statement 5.5: Patients who undergo treatment de-escalation should be monitored for relapse, especially in the initial period after discontinuation or reduction in dose (Evidence level 3, 5) | Voting agreement rate 46/47 (97.9%) |
During treatment de-escalation, patients should be regularly monitored for relapse, especially in the initial period (6–12 months) after de-escalation during which the risk of relapse is greatest [244, 257]. Despite this, the duration during which the risk of relapse is greatest following discontinuation differs depending on prior treatment. Although the timing of peak relapse for different therapies is difficult to specify because of variation among study characteristics and findings, the risk of relapse appears to be high in the early period after discontinuation of monotherapy compared with combination therapy. There is a lack of consistent evidence to recommend a single optimal protocol for disease monitoring. However, a combined approach including monitoring of symptoms, biomarkers (e.g., CRP/FC), and/or endoscopy/imaging has been recommended by a recent European expert consensus panel [244]. A subanalysis of the STORI population found that elevated levels of CRP and FC were associated with an increased risk of short-term relapse following infliximab discontinuation [258]. Similarly, in a prospective cohort study of children with CD, FC levels > 250 μg/g accurately predicted clinical flares within 3 months [259]. As a consequence, expert consensus guidelines have suggested that CRP or FC should be used to monitor patients following discontinuation with elevated levels used as a trigger for further examination [244].
A systematic review and meta-analysis among CD patients in deep remission found that the rate of recapture of remission was relatively high (75.4%) after treatment was reinitiated [260].
Conclusions
As T2T strategies become more prevalent in CD, it is important to identify clear indications for the escalation and de-escalation of treatment based on patient response. The current consensus document provides a framework and guidance for clinicians who are making these decisions in clinical practice and are specifically focused on making these decisions in different clinical scenarios, such as after surgery or in patients with perianal disease.
References
Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–106.
Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–38.
Terdiman JP, Gruss CB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1459–63.
Bernstein CN, Eliakim A, Fedail S, et al. World Gastroenterology Organisation global guidelines inflammatory bowel disease: update August 2015. J Clin Gastroenterol. 2016;50:803–18.
Gomollon F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25.
Peyrin-Biroulet L, Bouhnik Y, Roblin X, et al. French national consensus clinical guidelines for the management of Crohn’s disease. Dig Liver Dis. 2017;49:368–77.
Ramakrishna BS, Makharia GK, Ahuja V, et al. Indian Society of Gastroenterology consensus statements on Crohn’s disease in India. Indian J Gastroenterol. 2015;34:3–22.
Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8:1179–207.
Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14:4–22.
Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2020.
Oxford University Centre for Evidence-Based Medicine. Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). 2009. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009. Accessed April to July.
Assa A, Matar M, Turner D, et al. Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn’s disease compared with reactive monitoring. Gastroenterology. 2019;157:985-96.e2.
Colombel JF, D’Haens G, Lee WJ, et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2019;12:12.
Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–89.
Derwa Y, Gracie DJ, Hamlin PJ, et al. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:389–400.
Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn’s disease—algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51.
Dreesen E, Van Stappen T, Ballet V, et al. Anti-infliximab antibody concentrations can guide treatment intensification in patients with Crohn’s disease who lose clinical response. Aliment Pharmacol Ther. 2018;47:346–55.
Dubinsky MC, Rosh J, Faubion WA Jr, et al. Efficacy and safety of escalation of adalimumab therapy to weekly dosing in pediatric patients with Crohn’s disease. Inflamm Bowel Dis. 2016;22:886–93.
Duveau N, Nachury M, Gerard R, et al. Adalimumab dose escalation is effective and well tolerated in Crohn’s disease patients with secondary loss of response to adalimumab. Dig Liver Dis. 2017;49:163–9.
Ghaly S, Costello S, Beswick L, et al. Dose tailoring of anti-tumour necrosis factor-alpha therapy delivers useful clinical efficacy in Crohn disease patients experiencing loss of response. Intern Med J. 2015;45:170–7.
Gils A. Combining therapeutic drug monitoring with biosimilars, a strategy to improve the efficacy of biologicals for treating inflammatory bowel diseases at an affordable cost. Dig Dis. 2017;35:61–8.
Gonczi L, Kurti Z, Rutka M, et al. Drug persistence and need for dose intensification to adalimumab therapy; the importance of therapeutic drug monitoring in inflammatory bowel diseases. BMC Gastroenterol. 2017;17:97.
Guidi L, Pugliese D, Tonucci TP, et al. Therapeutic drug monitoring is more cost-effective than a clinically based approach in the management of loss of response to infliximab in inflammatory bowel disease: an observational multicentre study. J Crohns Colitis. 2018;12:1079–88.
Hendler SA, Cohen BL, Colombel JF, et al. High-dose infliximab therapy in Crohn’s disease: clinical experience, safety, and efficacy. J Crohns Colitis. 2015;9:266–75.
Kalla R, Kennedy NA, Ventham NT, et al. Serum calprotectin: a novel diagnostic and prognostic marker in inflammatory bowel diseases. Am J Gastroenterol. 2016;111:1796–805.
Kang B, Choi SY, Kim HS, et al. Mucosal healing in paediatric patients with moderate-to-severe luminal Crohn’s disease under combined immunosuppression: escalation versus early treatment. J Crohns Colitis. 2016;10:1279–86.
Lindsay JO, Armuzzi A, Gisbert JP, et al. Indicators of suboptimal tumor necrosis factor antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2017;49:1086–91.
Ma C, Huang V, Fedorak DK, et al. Adalimumab dose escalation is effective for managing secondary loss of response in Crohn’s disease. Aliment Pharmacol Ther. 2014;40:1044–55.
Michopoulos S, Paspatis G, Triantafyllou K, et al. A multicenter, prospective, observational study of the long-term outcomes of Crohn’s disease patients under routine care management in Greece. Ann. 2018;31:583–92.
Minar P, Saeed SA, Afreen M, et al. Practical use of infliximab concentration monitoring in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2016;62:715–22.
Motoya S, Watanabe M, Wallace K, et al. Efficacy and safety of dose escalation to adalimumab 80 mg every other week in Japanese patients with Crohn’s disease who lost response to maintenance therapy. Inflamm Intest Dis. 2018;2:228–35.
Nagata Y, Esaki M, Umeno J, et al. Therapeutic strategy for Crohn’s disease with a loss of response to infliximab: a single-center retrospective study. Digestion. 2015;91:50–6.
Peeters H, Louis E, Baert F, et al. Efficacy of switching to infliximab in patients with Crohn’s disease with loss of response to adalimumab. Acta Gastroenterol Belg. 2018;81:15–21.
Qiu X, Feng JR, Chen LP, et al. Efficacy and safety of autologous hematopoietic stem cell therapy for refractory Crohn’s disease: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96: e7381.
Qiu Y, Li MY, Feng T, et al. Systematic review with meta-analysis: the efficacy and safety of stem cell therapy for Crohn’s disease. Stem Cell Res Ther. 2017;8:136.
Restellini S, Chao CY, Lakatos PL, et al. Therapeutic drug monitoring guides the management of Crohn’s patients with secondary loss of response to adalimumab. Inflamm Bowel Dis. 2018;24:1531–8.
Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol. 2014;109:1250–6.
Samaan MA, Birdi S, Morales MS, et al. Effectiveness of vedolizumab dose intensification to achieve inflammatory bowel disease control in cases of suboptimal response. Frontline Gastroenterol. 2019;11:188–93.
Steenholdt C, Bendtzen K, Brynskov J, et al. Changes in serum trough levels of infliximab during treatment intensification but not in antiinfliximab antibody detection are associated with clinical outcomes after therapeutic failure in Crohn’s disease. J Crohns Colitis. 2015;9:238–45.
Steenholdt C, Brynskov J, Thomsen OO, et al. Individualized therapy is a long-term cost-effective method compared to dose intensification in crohn’s disease patients failing infliximab. Dig Dis Sci. 2015;60:2762–70.
Suzuki T, Mizoshita T, Sugiyama T, et al. Adalimumab dose-escalation therapy is effective in refractory Crohn’s disease patients with loss of response to adalimumab, especially in cases without previous infliximab treatment. Case Rep Gastroenterol. 2019;13:37–49.
Taxonera C, Olivares D, Mendoza JL, et al. Need for infliximab dose intensification in Crohn’s disease and ulcerative colitis. World J Gastroenterol. 2014;20:9170–7.
Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320-9.e3.
Vavricka SR, Radivojevic S, Manser CN, et al. Addressing current treatment challenges in Crohn’s disease in real life: a physician’s survey. Dig Liver Dis. 2014;46:1066–71.
Verstockt B, Moors G, Bian S, et al. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn’s disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther. 2018;48:731–9.
Viazis N, Koukouratos T, Anastasiou J, et al. Azathioprine discontinuation earlier than 6 months in Crohn’s disease patients started on anti-TNF therapy is associated with loss of response and the need for anti-TNF dose escalation. Eur J Gastroenterol Hepatol. 2015;27:436–41.
Hoekman DR, Stibbe JA, Baert FJ, et al. Long-term outcome of early combined immunosuppression versus conventional management in newly diagnosed Crohn’s disease. J Crohns Colitis. 2018;12:517–24.
Kósa F, Kunovszki P, Borsi A, et al. Anti-TNF dose escalation and drug sustainability in Crohn’s disease: data from the nationwide administrative database in Hungary. Dig Liver Dis. 2020;52:274–80.
Pallotta N, Vincoli G, Pezzotti P, et al. A risk score system to timely manage treatment in Crohn’s disease: a cohort study. BMC Gastroenterol. 2018;18:164.
Vavricka SR, Schoepfer A, Scharl M, et al. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1982–92.
Long MD. Assessing extraintestinal manifestations of IBD. Gastroenterol Hepatol (N Y). 2015;11:118–20.
Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512–30.
Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–44.
Kishi M, Hirai F, Takatsu N, et al. A review on the current status and definitions of activity indices in inflammatory bowel disease: how to use indices for precise evaluation. J Gastroenterol. 2022;57:246–66.
D’Haens G, Vermeire S, Lambrecht G, et al. Increasing infliximab dose based on symptoms, biomarkers, and serum drug concentrations does not increase clinical, endoscopic, and corticosteroid-free remission in patients with active luminal Crohn’s disease. Gastroenterology. 2018;154:1343-51.e1.
Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63:919–27.
D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–7.
Panaccione R, Sandborn WJ, D’Haens G, et al. Clinical benefit of long-term adalimumab treatment in patients with Crohn’s disease following loss of response or intolerance to infliximab: 96-week efficacy data from GAIN/ADHERE trials. J Crohns Colitis. 2018;12:930–8.
Lewis JD, Rutgeerts P, Feagan BG, et al. Correlation of stool frequency and abdominal pain measures with simple endoscopic score for Crohn’s disease. Inflamm Bowel Dis. 2020;26:304–13.
Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386:1825–34.
Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8:357–63.
Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514.
Derwa Y, Williams CJM, Sood R, et al. Factors affecting clinical decision-making in inflammatory bowel disease and the role of point-of-care calprotectin. Therap. 2018;11:1756283–17744739.
Jones GR, Fasci-Spurio F, Kennedy NA, et al. Faecal calprotectin and magnetic resonance enterography in ileal Crohn’s disease: Correlations between disease activity and long-term follow-up. J Crohns Colitis. 2019;13:442–50.
Nguyen GC, Boland K, Afif W, et al. Modified Delphi process for the development of choosing wisely for inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:858–65.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9.
Christensen B, Erlich J, Gibson PR, et al. Histologic healing is more strongly associated with clinical outcomes in ileal Crohn’s disease than endoscopic healing. Clin Gastroenterol Hepatol. 2020;18:2518-25.e1.
Dal Buono A, Roda G, Argollo M, et al. Treat to target or “treat to clear” in inflammatory bowel diseases: one step further? Expert Rev Gastroenterol Hepatol. 2020;14:807–17.
Prideaux L, Kamm MA, De Cruz PP, et al. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012;27:1266–80.
Hisabe T, Hirai F, Matsui T, et al. Evaluation of diagnostic criteria for Crohn’s disease in Japan. J Gastroenterol. 2014;49:93–9.
Saygili F, Saygili SM, Oztas E. Examining the whole bowel, double balloon enteroscopy: indications, diagnostic yield and complications. World J Gastrointest Endosc. 2015;7:247–52.
Takenaka K, Kitazume Y, Fujii T, et al. Objective evaluation for treat to target in Crohn’s disease. J Gastroenterol. 2020;55:579–87.
Takenaka K, Ohtsuka K, Kitazume Y, et al. Utility of magnetic resonance enterography for small bowel endoscopic healing in patients with Crohn’s disease. Am J Gastroenterol. 2018;113:283–94.
Saibeni S, Rondonotti E, Iozzelli A, et al. Imaging of the small bowel in Crohn’s disease: a review of old and new techniques. World J Gastroenterol. 2007;13:3279–87.
Solem CA, Loftus EV Jr, Fletcher JG, et al. Small-bowel imaging in Crohn’s disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255–66.
Nakamura M, Yamamura T, Maeda K, et al. Validity of capsule endoscopy in monitoring therapeutic interventions in patients with Crohn’s disease. JCM. 2018;7:29.
Miyazu T, Ishida N, Takano R, et al. Usefulness of the capsule endoscopy Crohn’s disease activity index in assessing the necessity of early additional treatment in patients with Crohn’s disease in clinical remission. Medicine (Baltimore). 2021;100: e26550.
Nishikawa T, Nakamura M, Yamamura T, et al. Lewis score on capsule endoscopy can predict the prognosis in patients with small bowel lesions of Crohn’s disease. J Gastroenterol Hepatol. 2021;36:1851–8.
Oliva S, Veraldi S, Cucchiara S, et al. Assessment of a new score for capsule endoscopy in pediatric Crohn’s disease (CE-CD). Endosc Int Open. 2021;9:E1480–90.
Tai FWD, Ellul P, Elosua A, et al. Panenteric capsule endoscopy identifies proximal small bowel disease guiding upstaging and treatment intensification in Crohn’s disease: a European multicentre observational cohort study. United EurGastroenterol J. 2021;9:248–55.
D’Inca R, Caccaro R. Measuring disease activity in Crohn’s disease: what is currently available to the clinician. Clin Exp Gastroenterol. 2014;7:151–61.
Manetta R, Capretti I, Belleggia N, et al. Magnetic resonance enterography (MRE) and ultrasonography (US) in the study of the small bowel in Crohn’s disease: state of the art and review of the literature. Acta Biomed. 2019;90:38–50.
Bruining DH, Zimmermann EM, Loftus EV Jr, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology. 2018;286:776–99.
Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–61.
Les A, Iacob R, Saizu R, et al. Bowel ultrasound: a non-invasive, easy to use method to predict the need to intensify therapy in inflammatory bowel disease patients. JGLD. 2021;30:462–9.
Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–85.
Esaki M, Matsumoto T, Watanabe K, et al. Use of capsule endoscopy in patients with Crohn’s disease in Japan: a multicenter survey. J Gastroenterol Hepatol. 2014;29:96–101.
Bungay H. Small bowel imaging in Crohn’s disease. Frontline Gastroenterol. 2012;3:39–46.
Gourtsoyiannis NC, Grammatikakis J, Papamastorakis G, et al. Imaging of small intestinal Crohn’s disease: comparison between MR enteroclysis and conventional enteroclysis. Eur Radiol. 2006;16:1915–25.
Leighton JA, Gralnek IM, Cohen SA, et al. Capsule endoscopy is superior to small-bowel follow-through and equivalent to ileocolonoscopy in suspected Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:609–15.
Masselli G, Vecchioli A, Gualdi GF. Crohn disease of the small bowel: MR enteroclysis versus conventional enteroclysis. Abdom Imaging. 2006;31:400–9.
Liverani E, Scaioli E, Digby RJ, et al. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol. 2016;22:1017–33.
Puylaert CA, Tielbeek JA, Bipat S, et al. Grading of Crohn’s disease activity using CT, MRI, US and scintigraphy: a meta-analysis. Eur Radiol. 2015;25:3295–313.
Serafin Z, Bialecki M, Bialecka A, et al. Contrast-enhanced ultrasound for detection of Crohn’s disease activity: systematic review and meta-analysis. J Crohns Colitis. 2016;10:354–62.
Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983–9.
Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–12.
Vuitton L, Marteau P, Sandborn WJ, et al. IOIBD technical review on endoscopic indices for Crohn’s disease clinical trials. Gut. 2016;65:1447–55.
Sipponen T, Nuutinen H, Turunen U, et al. Endoscopic evaluation of Crohn’s disease activity: comparison of the CDEIS and the SES-CD. Inflamm Bowel Dis. 2010;16:2131–6.
Kucharski M, Karczewski J, Mankowska-Wierzbicka D, et al. Usefulness of endoscopic indices in determination of disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2016;2016:7896478.
Klenske E, Bojarski C, Waldner M, et al. Targeting mucosal healing in Crohn’s disease: what the clinician needs to know. Therap Adv Gastroenterol. 2019;12:1756284819856865.
Novak G, Parker CE, Pai RK, et al. Histologic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev. 2017;7:CD012351.
Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–8.
Serada S, Fujimoto M, Ogata A, et al. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis. 2010;69:770–4.
Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: A systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–19 (quiz 20).
Chang S, Malter L, Hudesman D. Disease monitoring in inflammatory bowel disease. World J Gastroenterol. 2015;21:11246–59.
Nakamura S, Imaeda H, Nishikawa H, et al. Usefulness of fecal calprotectin by monoclonal antibody testing in adult Japanese with inflammatory bowel diseases: a prospective multicenter study. Intest Res. 2018;16:554–62.
Mumolo MG, Bertani L, Ceccarelli L, et al. From bench to bedside: fecal calprotectin in inflammatory bowel diseases clinical setting. World J Gastroenterol. 2018;24:3681–94.
El-Matary W, Abej E, Deora V, et al. Impact of fecal calprotectin measurement on decision-making in children with inflammatory bowel disease. Front. 2017;5:7.
Reinisch W, Panaccione R, Bossuyt P, et al. Association of biomarker cutoffs and endoscopic outcomes in crohn’s disease: a post hoc analysis from the CALM study. Inflamm Bowel Dis. 2020;26:1562–71.
Komaki Y, Kanmura S, Yutsudo K, et al. Infliximab therapy intensification based on endoscopic activity is related to suppress treatment discontinuation in patients with Crohn disease: a retrospective cohort study. Medicine (Baltimore). 2021;100: e24731.
Danese S, Vermeire S, D’Haens G, et al. Treat to target versus standard of care for patients with Crohn’s disease treated with ustekinumab (STARDUST): an open-label, multicentre, randomised phase 3b trial. Lancet Gastroenterol Hepatol. 2022;7:294–306.
Verdejo C, Hervias D, Roncero O, et al. Fecal calprotectin is not superior to serum C-reactive protein or the Harvey-Bradshaw index in predicting postoperative endoscopic recurrence in Crohn’s disease. Eur J Gastroenterol Hepatol. 2018;30:1521–7.
Dalal SR, Cohen RD. What to do when biologic agents are not working in inflammatory bowel disease patients. Gastroenterol Hepatol. 2015;11:657–65.
Fernandes SR, Bernardo S, Simoes C, et al. Proactive infliximab drug monitoring is superior to conventional management in inflammatory bowel disease. Inflamm Bowel Dis. 2019;27:27.
Burgess CJ, Reilly C, Steward-Harrison L, et al. Utility of proactive infliximab levels in paediatric Crohn’s disease. Arch Dis Child. 2019;104:251–5.
Choi SY, Kang B, Lee JH, et al. Clinical use of measuring trough levels and antibodies against infliximab in patients with pediatric inflammatory bowel disease. Gut Liver. 2017;11:55–61.
Melmed GY, Irving PM, Jones J, et al. Appropriateness of testing for anti-tumor necrosis factor agent and antibody concentrations, and interpretation of results. Clin Gastroenterol Hepatol. 2016;14:1302–9.
Papamichael K, Juncadella A, Wong D, et al. Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared with standard of care in patients with inflammatory bowel disease. J Crohns Colitis. 2019;13:976–81.
Roblin X, Marotte H, Leclerc M, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis. 2015;9:525–31.
Walker R, Kammermeier J, Vora R, et al. Azathioprine dosing and metabolite measurement in pediatric inflammatory bowel disease: does one size fit all? Ann. 2019;32:387–91.
Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut. 2018;67:818–26.
Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–34.
Nakase H, Matsumoto T, Watanabe K, et al. The shining DIAMOND for evidence-based treatment strategies for Crohn’s disease. J Gastroenterol. 2020;55:824–32.
Kamperidis N, Middleton P, Tyrrell T, et al. Impact of therapeutic drug level monitoring on outcomes of patients with Crohn’s disease treated with Infliximab: real world data from a retrospective single centre cohort study. Frontline Gastroenterol. 2019;10:330–6.
Mitrev N, Vande Casteele N, Seow CH, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;46:1037–53.
Scharnhorst V, Schmitz EMH, van de Kerkhof D, et al. A value proposition for trough level-based anti-TNFalpha drug dosing. Clin Chim Acta. 2019;489:89–95.
Kakuta Y, Kinouchi Y, Shimosegawa T. Pharmacogenetics of thiopurines for inflammatory bowel disease in East Asia: prospects for clinical application of NUDT15 genotyping. J Gastroenterol. 2018;53:172–80.
Odahara S, Uchiyama K, Kubota T, et al. A prospective study evaluating metabolic capacity of thiopurine and associated adverse reactions in Japanese patients with inflammatory bowel disease (IBD). PLoS ONE. 2015;10: e0137798.
Kakuta Y, Kawai Y, Okamoto D, et al. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. 2018;53:1065–78.
Kakuta Y, Izumiyama Y, Okamoto D, et al. High-resolution melt analysis enables simple genotyping of complicated polymorphisms of codon 18 rendering the NUDT15 diplotype. J Gastroenterol. 2020;55:67–77.
Banasiewicz T, Eder P, Rydzewska G, et al. Statement of the expert group on the current practice and prospects for the treatment of complex perirectal fistulas in the course of Crohn’s disease. Pol Przegl Chir. 2019;91:38–46.
Fumery M, Pariente B, Sarter H, et al. Long-term outcome of pediatric-onset Crohn’s disease: a population-based cohort study. Dig Liver Dis. 2019;51:496–502.
Osamura A, Suzuki Y. Fourteen-year anti-TNF therapy in Crohn’s disease patients: clinical characteristics and predictive factors. Dig Dis Sci. 2018;63:204–8.
Rayen J, Currie T, Gearry RB, et al. The long-term outcome of anti-TNF alpha therapy in perianal Crohn’s disease. Tech Coloproctol. 2017;21:119–24.
Yoon JY, Cheon JH, Park SJ, et al. Effects of perianal involvement on clinical outcomes in Crohn’s disease over 10 years. Gut Liver. 2018;12:297–305.
Zhao M, Lo BZS, Vester-Andersen MK, et al. A 10-year follow-up study of the natural history of perianal Crohn’s disease in a Danish population-based inception cohort. Inflamm Bowel Dis. 2019;25:1227–36.
Hellers G, Bergstrand O, Ewerth S, et al. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut. 1980;21:525–7.
Malian A, Riviere P, Bouchard D, et al. Pedictors of perianal fistula relapse in Crohn’s disease. Inflamm Bowel Dis. 2019;26:926–31.
Mahadev S, Young JM, Selby W, et al. Quality of life in perianal Crohn’s disease: what do patients consider important? Dis Colon Rectum. 2011;54:579–85.
Riss S, Schwameis K, Mittlbock M, et al. Sexual function and quality of life after surgical treatment for anal fistulas in Crohn’s disease. Tech Coloproctol. 2013;17:89–94.
Gionchetti P, Dignass A, Danese S, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 2: surgical management and special situations. J Crohns Colitis. 2017;11:135–49.
Bouchard D, Brochard C, Vinson-Bonnet B, et al. How to manage anal ulcerations and anorectal stenosis in Crohn’s disease: algorithm-based decision making: French National Working Group Consensus 2018. Tech Coloproctol. 2019;23:353–60.
Wallenhorst T, Brochard C, Le Balch E, et al. Anal ulcerations in Crohn’s disease: natural history in the era of biological therapy. Dig Liver Dis. 2017;49:1191–5.
Steinhart AH, Panaccione R, Targownik L, et al. Clinical practice guideline for the medical management of perianal fistulizing Crohn’s disease: the Toronto consensus. Inflamm Bowel Dis. 2019;25:1–13.
Sandborn WJ, Fazio VW, Feagan BG, et al. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125:1508–30.
Gecse KB, Bemelman W, Kamm MA, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–92.
Irvine EJ. Usual therapy improves perianal Crohn’s disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol. 1995;20:27–32.
Bouchard D, Pigot F, de Parades V, et al. Management of perianal fistulas in Crohn’s disease: a 2021 update of the French National Society of Coloproctology consensus. Tech Coloproctol. 2022;26:805–11.
Klag T, Goetz M, Stange EF, et al. Medical therapy of perianal Crohn’s disease. Viszeralmedizin. 2015;31:265–72.
Castano-Milla C, Chaparro M, Saro C, et al. Effectiveness of adalimumab in perianal fistulas in crohn’s disease patients naive to anti-TNF therapy. J Clin Gastroenterol. 2015;49:34–40.
Iwanczak BM, Ryzko J, Jankowski P, et al. Induction and maintenance infliximab therapy for the treatment of Crohn’s disease with perianal fistulas in children: Retrospective, multicenter study. Adv Clin Exp Med. 2016;25:523–30.
Ji CC, Takano S. Clinical efficacy of adalimumab versus infliximab and the factors associated with recurrence or aggravation during treatment of anal fistulas in Crohn’s disease. Intest Res. 2017;15:182–6.
Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–405.
Ruemmele FM, Rosh J, Faubion WA, et al. Efficacy of adalimumab for treatment of perianal fistula in children with moderately to severely active Crohn’s disease: Results from IMAgINE 1 and IMAgINE 2. J Crohns Colitis. 2018;12:1249–54.
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–85.
Davidov Y, Ungar B, Bar-Yoseph H, et al. Association of induction infliximab levels with clinical response in perianal Crohn’s disease. J Crohns Colitis. 2017;11:549–55.
El-Matary W, Walters TD, Huynh HQ, et al. Higher postinduction infliximab serum trough levels are associated with healing of fistulizing perianal Crohn’s disease in children. Inflamm Bowel Dis. 2019;25:150–5.
Strik AS, Lowenberg M, Buskens CJ, et al. Higher anti-TNF serum levels are associated with perianal fistula closure in Crohn’s disease patients. Scand J Gastroenterol. 2019;54:453–8.
Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther. 2017;45:933–40.
Van Assche G, Vanbeckevoort D, Bielen D, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn’s disease. Am J Gastroenterol. 2003;98:332–9.
Brochard C, Landemaine A, L’Heritier AM, et al. Anal fistulas in severe perineal Crohn’s disease: MRI assessment in the determination of long-term healing rates. Inflamm Bowel Dis. 2018;24:1612–8.
Hindryckx P, Jairath V, Zou G, et al. Development and validation of a magnetic resonance index for assessing fistulas in patients with Crohn’s disease. Gastroenterology. 2019;157(1233–44): e5.
Sostegni R, Daperno M, Scaglione N, et al. Review article: Crohn’s disease: monitoring disease activity. Aliment Pharmacol Ther. 2003;17(Suppl 2):11–7.
Panes J, Reinisch W, Rupniewska E, et al. Burden and outcomes for complex perianal fistulas in Crohn’s disease: systematic review. World J Gastroenterol. 2018;24:4821–34.
Lee MJ, Brown SR, Fearnhead NS, et al. How are we managing fistulating perianal Crohn’s disease? Results of a national survey of consultant gastroenterologists. Frontline Gastroenterol. 2018;9:16–22.
McCurdy JD, Parlow S, Dawkins Y, et al. Tumor necrosis factor inhibitors may have limited efficacy for complex perianal fistulas without luminal Crohn’s disease. Dig Dis Sci. 2019;65:1784–9.
Deng F, Xia P, Wu Z, et al. Perianal and luminal relapse following perianal surgical intervention in Crohn’s disease. Int J Gen Med. 2021;14:3387–96.
Nam K, Jung WB, Lee SB, et al. Predictors of reoperation for perianal fistula in Crohn’s disease. J Dig Dis. 2021;22:334–41.
Pearson DC, May GR, Fick GH, et al. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123:132–42.
Present DH, Korelitz BI, Wisch N, et al. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981–7.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Bouguen G, Siproudhis L, Gizard E, et al. Long-term outcome of perianal fistulizing Crohn’s disease treated with infliximab. Clin Gastroenterol Hepatol. 2013;11(975–81):e1-4.
Tozer P, Ng SC, Siddiqui MR, et al. Long-term MRI-guided combined anti-TNF-alpha and thiopurine therapy for Crohn’s perianal fistulas. Inflamm Bowel Dis. 2012;18:1825–34.
Bouchard D, Abramowitz L, Bouguen G, et al. Anoperineal lesions in Crohn’s disease: French recommendations for clinical practice [Review]. Tech Coloproctol. 2017;21:683–91.
Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019;68:1115–26.
Bettenworth D, Nowacki TM, Cordes F, et al. Assessment of stricturing Crohn’s disease: current clinical practice and future avenues. World J Gastroenterol. 2016;22:1008–16.
Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–84.
Crohn's and Colitis Foundation. Fact Sheet: intestinal complications. 2015.
Chang CW, Wong JM, Tung CC, et al. Intestinal stricture in Crohn’s disease. Intest Res. 2015;13:19–26.
Lenze F, Wessling J, Bremer J, et al. Detection and differentiation of inflammatory versus fibromatous Crohn’s disease strictures: prospective comparison of 18F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm Bowel Dis. 2012;18:2252–60.
Louis E. Epidemiology of the transition from early to late Crohn’s disease. Dig Dis. 2012;30:376–9.
Rieder F. Managing intestinal fibrosis in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2018;14:120–2.
Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018;67:53–60.
Takenaka K, Ohtsuka K, Kitazume Y, et al. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn’s disease. Gastroenterology. 2014;147(334–42): e3.
Takenaka K, Ohtsuka K, Kitazume Y, et al. Magnetic resonance evaluation for small bowel strictures in Crohn’s disease: comparison with balloon enteroscopy. J Gastroenterol. 2017;52:879–88.
Sagami S, Kobayashi T, Kikkawa N, et al. Combination of colonoscopy and magnetic resonance enterography is more useful for clinical decision making than colonoscopy alone in patients with complicated Crohn’s disease. PLoS ONE. 2019;14: e0212404.
Schulberg JD, Wright EK, Holt BA, et al. Magnetic resonance enterography for predicting the clinical course of Crohn’s disease strictures. J Gastroenterol Hepatol. 2020;35:980–7.
Greenup AJ, Bressler B, Rosenfeld G. Medical imaging in small bowel Crohn’s disease-computer tomography enterography, magnetic resonance enterography, and ultrasound: “which one is the best for what?” Inflamm Bowel Dis. 2016;22:1246–61.
Takabayashi K, Hosoe N, Kato M, et al. Significance of endoscopic deep small bowel evaluation using balloon-assisted enteroscopy for Crohn’s disease in clinical remission. J Gastroenterol. 2021;56:25–33.
Rieder F, Latella G, Magro F, et al. European Crohn’s and Colitis Organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn’s disease. J Crohns Colitis. 2016;10:873–85.
Bessissow T, Reinglas J, Aruljothy A, et al. Endoscopic management of Crohn’s strictures. World J Gastroenterol. 2018;24:1859–67.
Klag T, Wehkamp J, Goetz M. Endoscopic balloon dilation for Crohn’s disease-associated strictures. Clin Endosc. 2017;50:429–36.
Navaneethan U, Lourdusamy V, Njei B, et al. Endoscopic balloon dilation in the management of strictures in Crohn’s disease: a systematic review and meta-analysis of non-randomized trials. Surg Endosc. 2016;30:5434–43.
Bettenworth D, Bokemeyer A, Kou L, et al. Systematic review with meta-analysis: efficacy of balloon-assisted enteroscopy for dilation of small bowel Crohn’s disease strictures. Aliment Pharmacol Ther. 2020;52:1104–16.
Hirai F, Andoh A, Ueno F, et al. Efficacy of endoscopic balloon dilation for small bowel strictures in patients with Crohn’s disease: a nationwide, multi-centre, open-label, prospective cohort study. J Crohns Colitis. 2018;12:394–401.
Bettenworth D, Gustavsson A, Atreja A, et al. A pooled analysis of efficacy, safety, and long-term outcome of endoscopic balloon dilation therapy for patients with stricturing Crohn’s disease. Inflamm Bowel Dis. 2017;23:133–42.
Hirai F, Beppu T, Takatsu N, et al. Long-term outcome of endoscopic balloon dilation for small bowel strictures in patients with Crohn’s disease. Dig Endosc. 2014;26:545–51.
Bamba S, Sakemi R, Fujii T, et al. A nationwide, multi-center, retrospective study of symptomatic small bowel stricture in patients with Crohn’s disease. J Gastroenterol. 2020;55:615–26.
Buisson A, Chevaux JB, Allen PB, et al. Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2012;35:625–33.
Yamamoto T, Shimoyama T. Monitoring and detection of disease recurrence after resection for Crohn’s disease: the role of non-invasive fecal biomarkers. Expert Rev Gastroenterol Hepatol. 2017;11:899–909.
Lopes S, Andrade P, Afonso J, et al. Correlation between calprotectin and modified Rutgeerts score. Inflamm Bowel Dis. 2016;22:2173–81.
Lightner AL, Shen B. Perioperative use of immunosuppressive medications in patients with Crohn’s disease in the new “biological era.” Gastroenterol Rep (Oxf). 2017;5:165–77.
Regueiro M, Velayos FS, Greer JB, et al. American Gastroenterological Association Institute Technical review on the management of Crohn’s disease after surgical resection. Gastroenterology. 2017;152:277–95.
De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406–17.
Watanabe T, Sasaki I, Sugita A, et al. Interval of less than 5 years between the first and second operation is a risk factor for a third operation for Crohn’s disease. Inflamm Bowel Dis. 2012;18:17–24.
Shinagawa T, Hata K, Ikeuchi H, et al. Rate of reoperation decreased significantly after year 2002 in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2020;18(898–907): e5.
American Gastroenterological Association. American Gastroenterological Institute guideline on the management of Crohn’s disease after surgical resection: clinical decision support tool. Gastroenterology. 2017;152:276.
Barnes EL, Lightner AL, Regueiro M. Perioperative and postoperative management of patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18:1356–66.
Hall B, Holleran G, Chin JL, et al. A prospective 52 week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. J Crohns Colitis. 2014;8:1601–9.
Han ZM, Qiao WG, Ai XY, et al. Impact of capsule endoscopy on prevention of postoperative recurrence of Crohn’s disease. Gastrointest Endosc. 2018;87:1489–98.
Yamamoto T. Diagnosis and monitoring of posteroperative recurrence in Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2015;9:55–66.
Yung DE, Har-Noy O, Tham YS, et al. Capsule endoscopy, magnetic resonance enterography, and small bowel ultrasound for evaluation of postoperative recurrence in Crohn’s disease: Systematic review and meta-analysis. Inflamm Bowel Dis. 2017;24:93–100.
Martinez MJ, Ripolles T, Paredes JM, et al. Intravenous contrast-enhanced ultrasound for assessing and grading postoperative recurrence of Crohn’s disease. Dig Dis Sci. 2019;64:1640–50.
Goetz M. Endoscopic surveillance in inflammatory bowel disease. Visc Med. 2018;34:66–71.
Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–63.
Regueiro M, Kip KE, Schraut W, et al. Crohn’s disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflamm Bowel Dis. 2011;17:118–26.
Turvill J. Mapping of Crohn’s disease outcomes to faecal calprotectin levels in patients maintained on biologic therapy. Frontline Gastroenterol. 2014;5:167–75.
Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology. 2015;148(938–47): e1.
Yamamoto T, Shimoyama T, Umegae S, et al. Serial monitoring of faecal calprotectin for the assessment of endoscopic recurrence in asymptomatic patients after ileocolonic resection for Crohn’s disease: a long-term prospective study. Therap Adv Gastroenterol. 2016;9:664–70.
Tham YS, Yung DE, Fay S, et al. Fecal calprotectin for detection of postoperative endoscopic recurrence in Crohn’s disease: systematic review and meta-analysis. Therap Adv Gastroenterol. 2018;11:1756284818785571.
Axelrad JE, Joelson A, Green PHR, et al. Enteric infections are common in patients with flares of inflammatory bowel disease. Am J Gastroenterol. 2018;113:1530–9.
Yamamoto T, Spinelli A, Suzuki Y, et al. Risk factors for complications after ileocolonic resection for Crohn’s disease with a major focus on the impact of preoperative immunosuppressive and biologic therapy: a retrospective international multicentre study. United European Gastroenterol J. 2016;4:784–93.
Barber GE, Hendler S, Okafor P, et al. Rising incidence of intestinal infections in inflammatory bowel disease: a nationwide analysis. Inflamm Bowel Dis. 2018;24:1849–56.
Casanova MJ, Chaparro M, Garcia-Sanchez V, et al. Evolution after anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol. 2017;112:120–31.
Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn's disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70 e5 (quiz e31).
Gallego JC, Echarri A, Porta A, et al. Ileal Crohn’s disease: magnetic resonance enterography as a predictor of relapse after antiTNF discontinuation. United Eur Gastroenterol J. 2015;3:A237–8.
Kennedy NA, Warner B, Johnston EL, et al. Relapse after withdrawal from anti-TNF therapy for inflammatory bowel disease: an observational study, plus systematic review and meta-analysis. Aliment Pharmacol Ther. 2016;43:910–23.
Molander P, Farkkila M, Kemppainen H, et al. Long-term outcome of inflammatory bowel disease patients with deep remission after discontinuation of TNFalpha-blocking agents. Scand J Gastroenterol. 2017;52:284–90.
Targownik LE, Tennakoon A, Leung S, et al. Factors associated with discontinuation of anti-TNF inhibitors among persons with IBD: a population-based analysis. Inflamm Bowel Dis. 2017;23:409–20.
Casanova MJ, Chaparro M, Nantes Ó, et al. Clinical outcome after anti-tumour necrosis factor therapy discontinuation in 1000 patients with inflammatory bowel disease: the EVODIS long-term study. Aliment Pharmacol Ther. 2021;53:1277–88.
Song JH, Kang EA, Park SK, et al. Long-term Outcomes after the discontinuation of anti-tumor necrosis factor-α therapy in patients with inflammatory bowel disease under clinical remission: a Korean association for the study of intestinal disease multicenter study. Gut Liver. 2021;15:752–62.
Bouhnik Y, Lemann M, Mary JY, et al. Long-term follow-up of patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Lancet. 1996;347:215–9.
Lemann M, Mary JY, Colombel JF, et al. A randomized, double-blind, controlled withdrawal trial in Crohn’s disease patients in long-term remission on azathioprine. Gastroenterology. 2005;128:1812–8.
O’Donoghue DP, Dawson AM, Powell-Tuck J, et al. Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn’s disease. Lancet. 1978;312:955–7.
Sokol H, Seksik P, Nion-Larmurier I, et al. Current smoking, not duration of remission, delays Crohn’s disease relapse following azathioprine withdrawal. Inflamm Bowel Dis. 2010;16:362–3.
Vilien M, Dahlerup JF, Munck LK, et al. Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn’s disease: increased relapse rate the following year. Aliment Pharmacol Ther. 2004;19:1147–52.
Wenzl HH, Primas C, Novacek G, et al. Withdrawal of long-term maintenance treatment with azathioprine tends to increase relapse risk in patients with Crohn’s disease. Dig Dis Sci. 2015;60:1414–23.
Treton X, Bouhnik Y, Mary JY, et al. Azathioprine withdrawal in patients with Crohn’s disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol. 2009;7:80–5.
Panaccione R, Ghosh S. Optimal use of biologics in the management of Crohn’s disease. Therap Adv Gastroenterol. 2010;3:179–89.
Hisamatsu T, Kato S, Kunisaki R, et al. Withdrawal of thiopurines in Crohn’s disease treated with scheduled adalimumab maintenance: a prospective randomised clinical trial (DIAMOND2). J Gastroenterol. 2019;54:860–70.
Oussalah A, Chevaux JB, Fay R, et al. Predictors of infliximab failure after azathioprine withdrawal in Crohn’s disease treated with combination therapy. Am J Gastroenterol. 2010;105:1142–9.
Roblin X, Boschetti G, Williet N, et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: an open-label, prospective and randomised clinical trial. Aliment Pharmacol Ther. 2017;46:142–9.
Van Assche G, Magdelaine-Beuzelin C, D’Haens G, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861–8.
Doherty G, Katsanos KH, Burisch J, et al. European Crohn’s and Colitis Organisation topical review on treatment withdrawal [’exit strategies’] in inflammatory bowel disease. J Crohns Colitis. 2018;12:17–31.
Gisbert JP, Marin AC, Chaparro M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment Pharmacol Ther. 2015;42:391–405.
Papamichael K, Vande Casteele N, Gils A, et al. Long-term outcome of patients with Crohn’s disease who discontinued infliximab therapy upon clinical remission. Clin Gastroenterol Hepatol. 2015;13:1103–10.
Papamichail K, Casteele NV, Hauenstein S, et al. Prediction of sustained remission after discontinuation of infliximab in patients with crohn's disease. Gastroenterology. 2014;146:S-457.
Ampuero J, Rojas-Feria M, Castro-Fernandez M, et al. Remission maintained by monotherapy after biological + immunosuppressive combination for Crohn’s disease in clinical practice. J Gastroenterol Hepatol. 2016;31:112–8.
Felice C, Pugliese D, Marzo M, et al. Deep remission improves clinical outcomes after infliximab discontinuation in inflammatory bowel diseases. United Eur Gastroenterol J. 2014;2:A221.
Bohn Thomsen S, Kiszka-Kanowitz M, Theede K, et al. Optimized thiopurine therapy before withdrawal of anti-tumour necrosis factor-alpha in patients with Crohn’s disease. Eur J Gastroenterol Hepatol. 2018;30:1155–8.
Ben-Horin S, Chowers Y, Ungar B, et al. Undetectable anti-TNF drug levels in patients with long-term remission predict successful drug withdrawal. Aliment Pharmacol Ther. 2015;42:356–64.
Helwig U, Lutter F, Koppka N, et al. Proposal for an anti-TNF-exit strategy based on trough serum level. Biologicals. 2017;47:81–5.
Dart RJ, Griffin N, Taylor K, et al. Reassessment of Crohn’s disease treated with at least 12 months of anti-TNF therapy: how likely is treatment withdrawal? Frontline Gastroenterol. 2014;5:176–82.
Kaymak T, Moriconi F, Niess JH, et al. Low discontinuation rate of infliximab treatment in steroid-dependent/refractory Crohn’s disease patients. Inflamm Intest Dis. 2018;2:171–9.
Bor R, Farkas K, Balint A, et al. Efficacy of combined anti-TNF-alpha and surgical therapy in perianal and enterocutaneous fistulizing Crohn’s disease–clinical observations from a tertiary Eastern European center. Scand J Gastroenterol. 2015;50:182–7.
Legue C, Brochard C, Bessi G, et al. Outcomes of perianal fistulising Crohn’s disease following anti-TNFalpha treatment discontinuation. Inflamm Bowel Dis. 2018;24:1107–13.
Frias Gomes C, Chapman TP, Satsangi J. De-escalation of medical therapy in inflammatory bowel disease. Curr Opin Pharmacol. 2020;55:73–81.
de Suray N, Salleron J, Vernier-Massouille G, et al. Close monitoring of CRP and fecal calprotectin levels to predict relapse in Crohn's disease patients. A sub-analysis of the STORI study. J Crohn's Colitis. 2012;6:S118-S9.
Foster AJ, Smyth M, Lakhani A, et al. Consecutive fecal calprotectin measurements for predicting relapse in pediatric Crohn’s disease patients. World J Gastroenterol. 2019;25:1266–77.
Zhang B, Gulati A, Alipour O, et al. Relapse from deep remission after therapeutic de-escalation in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2020;14:1413–23.
Hu H, Xiang C, Qiu C, et al. Discontinuation of scheduled infliximab in Crohn’s patients with clinical remission: a retrospective single-center study. Gastroenterol Res. 2017;10:92–9.
Molnar T, Lakatos PL, Farkas K, et al. Predictors of relapse in patients with Crohn’s disease in remission after 1 year of biological therapy. Aliment Pharmacol Ther. 2013;37:225–33.
Brooks AJ, Sebastian S, Cross SS, et al. Outcome of elective withdrawal of anti-tumour necrosis factor-alpha therapy in patients with Crohn’s disease in established remission. J Crohns Colitis. 2017;11:1456–62.
Torres J, Boyapati RK, Kennedy NA, et al. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology. 2015;149:1716–30.
Olivera P, Thiriet L, Luc A, et al. Treatment persistence for infliximab versus adalimumab in Crohn’s disease: a 14-year single-center experience. Inflamm Bowel Dis. 2017;23:976–85.
Kennedy NA, Kalla R, Warner B, et al. Thiopurine withdrawal during sustained clinical remission in inflammatory bowel disease: relapse and recapture rates, with predictive factors in 237 patients. Aliment Pharmacol Ther. 2014;40:1313–23.
Acknowledgements
Medical writing assistance was provided by Catherine Rees and Mark Snape of in Science Communications. Medical writing support was funded by AbbVie.
TRADE consensus group: Akira Andoh, Shigeki Bamba, Motohiro Esaki, Mikihiro Fujiya, Kitaro Futami, Keisuke Hata, Fumihito Hirai, Sakiko Hiraoka, Tadakazu Hisamatsu, Ryota Hokari, Shunji Ishihara, Soichiro Ishihara, Michio Itabashi, Yoichi Kakuta, Jun Kato, Shingo Kato, Takehiko Katsurada, Kazuya Kitamura, Kiyonori Kobayashi, Taku Kobayashi, Kazutaka Koganei, Atsuo Maemoto, Toshiyuki Matsui, Takayuki Matsumoto, Katsuyoshi Matsuoka, Minoru Matsuura, Satoshi Motoya, Masakazu Nagahori, Makoto Naganuma, Yuji Naito, Shiro Nakamura, Hiroshi Nakase, Haruhiko Ogata, Kazuichi Okazaki, Hirotake Sakuraba, Masayuki Saruta, Shinichiro Shinzaki, Ken Sugimoto, Akira Sugita, Yasuo Suzuki, Kenichi Takahashi, Tomohisa Takagi, Kento Takenaka, Ken Takeuchi, Kiichiro Tsuchiya, Tomoyuki Tsujikawa, Motoi Uchino, Fumiaki Ueno, Kenji Watanabe, Mamoru Watanabe, Takayuki Yamamoto, Kaoru Yokoyama, Atsushi Yoshida, Naoki Yoshimura.
Funding
This work was supported by AbbVie, which funded meeting attendance, provision of relevant materials, medical writing, and article processing charges.
Author information
Authors and Affiliations
Consortia
Contributions
HN and TH moderated the TRADE consensus group meetings. HN, TH, ME, FH, TK, KM, MM, MN, MS, KT, MU and KW attended the group meetings, contributed to statement generation, analyzed the voting results, had full access to all data, reviewed and revised all drafts of the manuscript, approved the final draft for submission, and had final responsibility for the decision to submit the manuscript for publication.
Corresponding authors
Ethics declarations
Conflict of interest
All authors received honoraria and administration costs from AbbVie to attend the meetings. Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors. Hiroshi Nakase has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Mylan EPD G.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd.; research grants from AbbVie GK, Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., PENTAX Medical, AYUMI Pharmaceutical Corporation; scholarship grants from AbbVie GK, EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Kayaku Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd. Motohiro Esaki has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for Mitsubishi Tanabe Pharma Corporation, AbbVie GK, Janssen Pharmaceutical K.K., EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd.; scholarship grants from Mitsubishi Tanabe Pharma Corporation, EA Pharma Co., Ltd., AbbVie GK, Takeda Pharmaceutical Co., Ltd. Fumihito Hirai has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, EA Pharma Co., Ltd., Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd.; research grants from Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., and AbbVie GK; scholarship grants from AbbVie GK, EA Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation. Taku Kobayashi has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc.; research grants from AbbVie GK, Activaid, Alfresa Pharma Corporation, JMDC, Gilead Sciences, Nippon Kayaku Co., Ltd., Eli Lilly Japan K.K., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Google Asia Pacific Pty. Ltd.; scholarship grants from Mitsubishi Tanabe Pharma Corporation, Zeria Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd.; and was an endowed chair for Otsuka Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., JIMRO Co., Ltd., AbbVie GK, Zeria Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., and Mochida Pharmaceutical Co., Ltd. Katsuyoshi Matsuoka has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., AbbVie GK, EA Pharma Co., Ltd., Pfizer Japan Inc., Mochida Pharmaceutical Co., Ltd.; research grants from Janssen Pharmaceutical K.K.; scholarship grants from Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd. Minoru Matsuura has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for Janssen Pharmaceutical K.K. Makoto Naganuma has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for Takeda Pharmaceutical Co., Ltd., AbbVie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, JIMRO Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Pfizer Japan Inc.; research grants from Mochida Pharmaceutical Co., Ltd.; scholarship grants from AbbVie GK, Mitsubishi Tanabe Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd. Masayuki Saruta has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Ltd., Takeda Pharmaceutical Co., Ltd., EA Pharma Co., Ltd.; fees for writing a pamphlet for EA Pharma Co., Ltd.; research grants from EPS Corporation; scholarship grants from Mochida Pharmaceutical Co., Ltd., Kiichiro Tsuchiya and Motoi Uchino have no conflicts of interest to declare. Kenji Watanabe has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, EA Pharma Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., research grants from EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., EP-CRSU Co., Ltd., scholarship grants from AbbVie GK, EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, JIMRO Co., Ltd., Nippon Kayaku Co., Ltd., and has been an endowed chair for AbbVie GK, EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Zeria Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Otsuka Pharmaceutical Factory, Inc., Asahi Kasei Medical Co., Ltd., Mochida Pharmaceutical Co., Ltd. Tadakazu Hisamatsu has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for EA Pharma Co. Ltd., AbbVie GK, Celgene K.K., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Nichi-Iko Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA Pharma Co. Ltd., Kyorin Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Japan Inc.; research grants from Alfresa Pharma Corporation, EA Pharma Co., Ltd.; scholarship grants from Mitsubishi Tanabe Pharma Corporation, EA Pharma Co., Ltd., AbbVie GK, JIMRO Co. Ltd., Zeria Pharmaceutical Co., Ltd., Daiichi-Sankyo, Kyorin Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Japan Inc., Mochida Pharmaceutical Co., Ltd. AbbVie, the sponsor, had no involvement in the development of the statements or the drafting of the current manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the TRADE consensus group are listed in “Acknowledgements”.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakase, H., Esaki, M., Hirai, F. et al. Treatment escalation and de-escalation decisions in Crohn’s disease: Delphi consensus recommendations from Japan, 2021. J Gastroenterol 58, 313–345 (2023). https://doi.org/10.1007/s00535-023-01958-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-023-01958-z