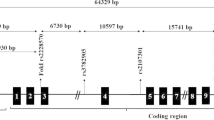
Abstract
Background
Polycystic ovarian syndrome (PCOS) is a prevailing endocrinopathy affecting a significant population of women of reproductive age across the globe. A myriad set of complex intertwined factors ranging from etiological, genetic, and epigenetic reasons cause this disorder. Out of the different factors, vitamin D shows an imperative aspect in health and fertility of women with polycystic ovary syndrome (PCOS). The importance of vitamin D is facilitated by vitamin D receptor (VDR), a ligand-dependent transcription factor in the steroid/ thyroid hormone receptor superfamily that controls the pleiotropic biological properties of vitamin D.
Purpose
The purpose of this study was to evaluate the role of promoter methylation of the VDR gene, a transcription factor with numerous biological utilities, with its relative expression and clinico-pathological findings and outcomes.
Methodology
A total of 200 blood samples were collected, 100 from PCOS case subjects, and 100 from the normal healthy controls respectively, which were assessed by qRT-PCR for determining the expression summary. MS-PCR technique was used for analyzing the promoter methylation status of the VDR gene. Blood samples were withdrawn, respectively, for each case and the control study separately experimented for different stages for the given study, of which estimation of vitamin D was also a part.
Results
In this test-versus-control study, first, the promoter methylation status of VDR gene was identified which was found more prominent i.e., hyper-methylation of the VDR gene was identified in 84 cases (84%), and in the normal healthy controls, it was found (62%). The promoter methylation status of the VDR gene has remarkably shown the results with a significant difference (p value < 0.0001*). Second, the expression analysis of VDR gene was found to be strongly downregulated in majority (64%) of PCOS case samples analyzed by means fold change of 0.8743 (± 0.06466) (p value 0.0054**). This result is, therefore, indicative of VDR gene role in PCOS pathogenesis as the said gene is downregulated. Moreover, compared to the vitamin D parameter, hyper-methylation and expression analysis of the VDR promoter gene were found to correspond to some associations with PCOS. Certain case-and-control study analyses showed that patients with normal vitamin D levels showed less indicative effects of PCOS and vice versa.
Conclusion
Our study, being exclusive from Kashmir, one of the foremost specified that VDR confirms anomalous methylation configuration in PCOS with subsequent downregulation in the gene expression i.e., there is an inverse correlation among VDR gene expression (downregulated) and methylation status (hyper-methylated) from the conclusion of our PCOS case-versus-control study.
Graphical abstract







Similar content being viewed by others
Data availability
This work has been published in our earlier artcile titled “Clinical profiling of Polycystic Ovary Syndrome patients in Kashmir Population”. The article details can be checked from: [http://www.matrixscipharma.org IP: 246.246.250.214] DOI: https://doi.org/10.4103/mtsp.mtsp_4_22.
Abbreviations
- PCOS:
-
Polycystic ovary syndrome
- VDR:
-
Vitamin D receptor
- RT-PCR:
-
Real-time polymerase chain reaction
- IR:
-
Insulin resistance
References
Zargar AH, Gupta VK, Wani AI, Masoodi SR, Bashir MI, Laway BA, Ganie MA, Salahudin M (2005) Prevalence of ultrasonically proved polycystic ovaries in North Indian women with Type 2 diabetes mellitus. Re-prod Biol Endocrinol 3:35
Lauritsen MP, Bentzen JG, Pinborg A et al (2014) The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Hum Reprod 29:791–801
Diamanti-Kandarakis E, Kouli CR, Bergiele AT et al (1999) A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 84:4006–4011
Franks S, McCarthy MI, Hardy K (2006) Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl 29:278–285
Toosy S, Sodi R, Pappachan JM (2018) Lean polycystic ovary syndrome (PCOS): an evidence-based practical approach. J Diabetes Metab Disord 17:277–285. https://doi.org/10.1007/s40200-018-0371-5
Gambineri A, Repaci A, Patton L, Grassi I, Pocognoli P, Cognigni GE et al (2009) Prominent role of low HDL-cholesterol in explaining the high prevalence of the metabolic syndrome in polycystic ovary syndrome. Nutr Metab Cardiovasc Dis 19:797–804. https://doi.org/10.1016/j.numecd.2009.01.007
Gambineri A, Patton L, Altieri P, Pagotto U, Pizzi C, Manzoli L et al (2012) Polycystic ovary syndrome is a risk factor for type 2 diabetes: results from a long-term prospective study. Diabetes 61:2369–2374. https://doi.org/10.2337/db11-1360
Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E (2015) American Association of Clinical Endocrinologists, American College of Endocrinology, and androgen excess and PCOS society disease state clinical review: a guide to the best practices in the evaluation and treatment of polycystic ovary syndrome Part 2. Endocr Pract 21:1415–1426. https://doi.org/10.4158/EP15748.DSCPT2
Doh E, Mbanya A, Kemfang-Ngowa JD, Dohbit S, Tchana-Sinou M, Foumane P et al (2016) The relationship between adiposity and insulin sensitivity in African women living with polycystic ovarian syndrome: a clamp study. Int J Endocrinol 2016:9201701. https://doi.org/10.1155/2016/9201701
Pasquali R, Gambineri A, Pagotto U (2006) The impact of obesity on reproduction in women with polycystic ovary syndrome. Br J Obstet Gynecol 113:1148–1159. https://doi.org/10.1111/j.1471-0528.2006.00990.x
Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C et al (1995) Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 10:2107–2111. https://doi.org/10.1093/oxfordjournals.humrep.a136243
Kahn CR (1994) Insulin action, diabetogenes, and the cause of Type II diabetes. Diabetes 43:1066–1084
Diamanti-Kandarakis E, Dunaif A (2012) Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33(6):981–1030. https://doi.org/10.1210/er.2011-1034
Wehr E, Moller R, Horejsi R, Giuliani A, Kopera D, Schweighofer N et al (2009) Subcutaneous adipose tissue topography and metabolic disturbances in polycystic ovary syndrome. Wien Klin Wochenschr 121:262–269
Studzinski G, McLane J, Uskokovic M (1993) Signaling pathways for vitamin D-induced differentiation: implications for therapy of proliferative and neoplastic diseases. Crit Rev Eukaryot Gene Expr 3:279–312
Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M (2008) Vitamin D, and human health: lessons from vitamin D receptor null mice. Endocr Rev 29(6):726–776. https://doi.org/10.1210/er.2008-0004
Thomson RL, Spedding S, Buckley JD (2012) Vitamin D in the etiology and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 77:343–350
Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B (2011) Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol 164(5):741–749. https://doi.org/10.1530/EJE-11-0134
Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338(2):143–156. https://doi.org/10.1016/j.gene.2004.05.014
Du H, Daftary GS, Lalwani SI, Taylor HS (2005) Direct regulation of HOXA10 by, 25-(OH)2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol 19:2222–2233
Parikh G, Varadinova M, Suwandhi P, Araki T, Rosenwaks Z, Poretsky L, Seto-Young D (2010) Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res 42:754–757
Irani M, Merhi Z (2014) Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril 102:460–469
Alvarez J, Ashraf A (2010) Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol 2010:351–385
Suguna SA, Nandal DH, Kamble SU, Bharatha AM, Kunkulol RA (2014) Genomic DNA isolation from human whole blood samples by non-enzymatic salting out method. Int J Pharm Sci 6(6):198–199
Nowak A, Majsterek I, Przybyłowska-Sygut K, Pytel D, Szymanek K, Szaflik J, Szaflik JP (2015) Analysis of the expression and polymorphism of APOE, HSP, BDNF, and GRIN2B genes associated with the neurodegeneration process in the pathogenesis of primary open-angle glaucoma. BioMed Res Int 2015:258281
Afshan FU, Masood A, Nissar B, Chowdri NA, Naykoo NA, Majid M, Ganai BA (2021) Promoter hypermethylation regulates vitamin D receptor (VDR) expression in the colorectal cancer—a study from Kashmir valley. Cancer Genet 252–253:96–106
Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, Obermayer-Pietsch B (2009) Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol 161:575–582
Grzesiak M (2020) Vitamin D3 action within the ovary-an updated review. Physiol Res 69:371–378
He C, Lin Z, Robb SW, Ezeamama AE (2015) Serum Vitamin D levels and polycystic ovary syndrome: a systematic review and meta-analysis. Nutrients 7:4555–4577
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007) Review: the role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029
Lerchbaum E (2012) Obermayer-Pietsch B, Vitamin D, and fertility: a systematic review. Eur J Endocrinol 166:765–778
Zhou F, Xing Y, Cheng T, Yang L, Ma H (2022) Exploration of hub genes involved in PCOS using biological informatics methods. Medicine 101(40):e30905. https://doi.org/10.1097/MD.0000000000030905
Kuramoto N, Nomura K, Kohno D et al (2021) Role of PDK1 in skeletal muscle hypertrophy induced by mechanical load. Sci Rep 11:3447
Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG et al (2009) Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet 280:559–563
Nandi A, Sinha N, Ong E, Sonmez H, Poretsky L (2016) Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Investig 25:15–28
Mahmoudi T, Majidzadeh-A K, Farahani H, Mirakhorli M, Dabiri R, Nobakht H et al (2015) Association of vitamin D receptor gene variants with polycystic ovary syndrome: a case–control study. Int J Reprod BioMed 13:793–800
Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A (2015) Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr 34:586–592
Foroozanfard F, Talebi M, Samimi M, Mehrabi S, Badehnoosh B, Jamilian M et al (2017) Effect of two different doses of vitamin D supplementation on metabolic profiles of insulin-resistant patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res 49:612–617
Fariba R, Aidin M, Shemirani AI, Mahmoudi T, Mohsen V, Nikzamir A et al (2010) Influence of gene variants related to calcium homeostasis on biochemical parameters of women with polycystic ovary syndrome. J Assist Reprod Genet 28:225–232
Zadeh-Vakili A, Ramezani Tehrani F, Daneshpour MS, Zarkesh M, Saadat N, Azizi F (2013) Genetic polymorphism of vitamin D receptor gene affects the phenotype of PCOS. Gene 515:193–196
Zhu H, Wang X, Shi H, Su S, Harshfield GA, Gutin B, Snieder H, Dong Y (2013) A genome-wide methylation study of severe vitamin D deficiency in African American adolescents. J Pediatr 162:1004-1009 e100
Zhou Y, Zhao LJ, Xu X, Ye A, Travers-Gustafson D, Zhou B, Wang HW, Zhang W, Lee Hamm L, Deng HW, Recker RR, Lappe JM (2014) DNA methylation levels of CYP2R1 and CYP24A1 predict vitamin D response variation. J Steroid Biochem Mol Biol 144(Pt A):207e14
Shahmoradi A, Aghaei A, Ghaderi K, JafarRezaei M, Azarnezhad A (2022) A meta-analysis of the association of ApaI, BsmI, FokI, and TaqI polymorphisms in the vitamin D receptor gene with the risk of polycystic ovary syndrome in the Eastern Mediterranean Regional Office population. Int J Reprod Biomed 20(6):433–446. https://doi.org/10.18502/ijrm.v20i6.11439
Ashraf A, Singh R, Mir S (2022) Clinical profiling of polycystic ovary syndrome patients in Kashmir population. Matrix Sci Pharma 6:23–33
Acknowledgements
The authors are highly thankful for the support and amenities provided by the Centre of Research for Development (CORD) at the University of Kashmir, Srinagar and the Department of Endocrinology at Superspeciality Hospital Srinagar, Kashmir. A. Ashraf acknowledges support from Shri Venkateshwara University for carrying out Ph.D. research on PCOS, from subject Biochemistry under the Department of Applied Sciences.
Funding
This research didn’t receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Professor (Dr.) R. Singh and A. Ashraf held the frame responsibility of the present study; A. Ashraf conducted the research, collected the patient data and information, composed the paper work and executed the statistical data analysis. Professor (Dr.) Bashir Ahmad Ganai held the main responsibility of providing the work understanding, including the theory understanding, bench work, methodological contributions and all lab work data to accomplish the practical portion of the research work. Dr. Shahnawaz Mir held the responsibility over guidance and compiling of clinico-pathological data of the patients in his presence. The intellectual content of this article was evaluated and analyzed decisively by Professor R. Singh, Professor B.A. Ganai and Dr. S. Mir. The major concern for the concluding work of this article was carried on by A. Ashraf, Professor R. Singh, Professor B.A. Ganai and Dr. S. Mir. The prime concern and aim of this research was to evaluate and perceive the study of PCOS genetically, epigenetically as well and clinico-pathologically with respect to vitamin D, so as to understand this disease over higher perspectives, which can be beneficial to the society in coming future and also to lead a study finding out if there can be some more and accurate diagnostic criteria of PCOS in near future.
Corresponding author
Ethics declarations
Conflict of interest
There has been no conflict of interest from any of the authors.
Ethics approval
The present research work was approved by the Institutional Ethics Committee (IEC) of GMC, Srinagar under Ref. No. 142/ETH/GMC/ICM, 2019.
Consent to participate
All the participants had given their written informed consent in a written in-formed consent letter. All methods were carried out in accordance with relevant guidelines and regulations.
Consent of publication
There was no need of publication consent as the article contained no personal or individual information, audio, or video.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashraf, A., Singh, R., Ganai, B.A. et al. Hypermethylation and down-regulation of vitamin D receptor (VDR) as contributing factors for polycystic ovary syndrome (PCOS): a case–control study from Kashmir, North India. Arch Gynecol Obstet 309, 1091–1100 (2024). https://doi.org/10.1007/s00404-023-07326-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07326-9