Abstract
Purpose
The liver-expressed antimicrobial peptide 2 (LEAP2) is a newly recognized peptide hormone that acts via the growth hormone secretagogue receptor (GHSR) blunting the effects of ghrelin and displaying ghrelin-independent actions. Since the implications of LEAP2 are beginning to be elucidated, we investigated if plasma LEAP2 concentration varies with feeding status or sex and whether it is associated with glucose metabolism and appetite sensations.
Methods
We performed a single test meal study, in which plasma concentrations of LEAP2, ghrelin, insulin and glucose as well as visual analogue scales for hunger, desire to eat, prospective food consumption, fullness were assessed before and 60 min after breakfast in 44 participants (n = 21 females) with normal weight (NW) or overweight/obesity (OW/OB).
Results
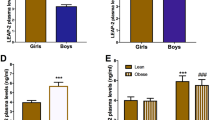
Pre-prandial plasma LEAP2 concentration was ~ 1.6-fold higher whereas ghrelin was ~ 2.0-fold lower in individuals with OW/OB (p < 0.001) independently of sex. After adjusting for body mass index (BMI) and sex, pre-prandial plasma LEAP2 concentration displayed a direct relationship with BMI (β: 0.09; 95%CI: 0.05, 0.13; p < 0.001), fat mass (β: 0.05; 95%CI: 0.01, 0.09; p = 0.010) and glycemia (β: 0.24; 95%CI: 0.05, 0.43; p = 0.021), whereas plasma ghrelin concentration displayed an inverse relationship with BMI and fat mass but not with glycemia. Postprandial plasma LEAP2 concentration increased ~ 58% in females with OW/OB (p = 0.045) but not in females with NW or in males. Pre-prandial plasma LEAP2 concentration displayed an inverse relationship with hunger score (β: − 11.16; 95% CI: − 18.52, − 3.79; p = 0.004), in a BMI-, sex- and ghrelin-independent manner.
Conclusions
LEAP2 emerges as a key hormone implicated in the regulation of metabolism and appetite in humans.
Trial registration
The study was retrospectively registered in clinicaltrials.gov (April 2023). ClinicalTrials.gov Identifier: NCT05815641.




Similar content being viewed by others
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Abbreviations
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- GHSR:
-
Growth hormone secretagogue receptor
- HOMA:
-
Homeostasis model assessment for insulin resistance
- IQR:
-
Interquartile range
- LEAP2:
-
Liver-expressed antimicrobial peptide 2
- NW:
-
Normal weight
- OB:
-
Obesity
- OW:
-
Overweight
- VAS:
-
Visual analogue scales
References
Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273(5277):974–977. https://doi.org/10.1126/science.273.5277.974
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402(6762):656–660. https://doi.org/10.1038/45230
Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR (2000) The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141(11):4325–4328. https://doi.org/10.1210/endo.141.11.7873
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409(6817):194–198. https://doi.org/10.1038/35051587
Gray SM, Page LC, Tong J (2019) Ghrelin regulation of glucose metabolism. J Neuroendocrinol 31(7):e12705. https://doi.org/10.1111/jne.12705
Perello M, Dickson SL (2015) Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. J Neuroendocrinol 27(6):424–434. https://doi.org/10.1111/jne.12236
Ge X, Yang H, Bednarek MA, Galon-Tilleman H, Chen P, Chen M, Lichtman JS, Wang Y, Dalmas O, Yin Y, Tian H, Jermutus L, Grimsby J, Rondinone CM, Konkar A, Kaplan DD (2018) LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metab 27(2):461–469. https://doi.org/10.1016/j.cmet.2017.10.016
Krause A, Sillard R, Kleemeier B, Kluver E, Maronde E, Conejo-Garcia JR, Forssmann WG, Schulz-Knappe P, Nehls MC, Wattler F, Wattler S, Adermann K (2003) Isolation and biochemical characterization of LEAP-2, a novel blood peptide expressed in the liver. Protein Sci 12(1):143–152. https://doi.org/10.1110/ps.0213603
M’Kadmi C, Cabral A, Barrile F, Giribaldi J, Cantel S, Damian M, Mary S, Denoyelle S, Dutertre S, Peraldi-Roux S, Neasta J, Oiry C, Baneres JL, Marie J, Perello M, Fehrentz JA (2019) N-Terminal Liver-expressed antimicrobial peptide 2 (LEAP2) region exhibits inverse agonist activity toward the ghrelin receptor. J Med Chem 62(2):965–973. https://doi.org/10.1021/acs.jmedchem.8b01644
Islam MN, Mita Y, Maruyama K, Tanida R, Zhang W, Sakoda H, Nakazato M (2020) Liver-expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J Endocrinol 244(1):13–23. https://doi.org/10.1530/JOE-19-0102
Bayle M, Peraldi-Roux S, Gautheron G, Cros G, Oiry C, Neasta J (2022) Liver-Expressed Antimicrobial Peptide 2 antagonizes the insulinostatic effect of ghrelin in rat isolated pancreatic islets. Fundam Clin Pharmacol 36(2):375–377. https://doi.org/10.1111/fcp.12722
Cornejo MP, Castrogiovanni D, Schioth HB, Reynaldo M, Marie J, Fehrentz JA, Perello M (2019) Growth hormone secretagogue receptor signalling affects high-fat intake independently of plasma levels of ghrelin and LEAP2, in a 4-day binge eating model. J Neuroendocrinol 31(10):e12785. https://doi.org/10.1111/jne.12785
Andreoli MF, De Francesco PN, Perello M (2018) Gastrointestinal hormones controlling energy homeostasis and their potential role in obesity. In: Nillni E (ed) Textbook of energy balance, neuropeptide hormones, and neuroendocrine function. Springer, Cham
Hagemann CA, Jensen MS, Holm S, Gasbjerg LS, Byberg S, Skov-Jeppesen K, Hartmann B, Holst JJ, Dela F, Vilsboll T, Christensen MB, Holst B, Knop FK (2022) LEAP2 reduces postprandial glucose excursions and ad libitum food intake in healthy men. Cell Rep Med 3(4):100582. https://doi.org/10.1016/j.xcrm.2022.100582
Cornejo MP, Mustafa ER, Cassano D, Baneres JL, Raingo J, Perello M (2021) The ups and downs of growth hormone secretagogue receptor signaling. FEBS J. https://doi.org/10.1111/febs.15718
Fernandez G, Cabral A, De Francesco PN, Uriarte M, Reynaldo M, Castrogiovanni D, Zubiria G, Giovambattista A, Cantel S, Denoyelle S, Fehrentz JA, Tolle V, Schioth HB, Perello M (2022) GHSR controls food deprivation-induced activation of CRF neurons of the hypothalamic paraventricular nucleus in a LEAP2-dependent manner. Cell Mol Life Sci CMLS 79(5):277. https://doi.org/10.1007/s00018-022-04302-5
Mani BK, Puzziferri N, He Z, Rodriguez JA, Osborne-Lawrence S, Metzger NP, Chhina N, Gaylinn B, Thorner MO, Thomas EL, Bell JD, Williams KW, Goldstone AP, Zigman JM (2019) LEAP2 changes with body mass and food intake in humans and mice. J Clin Invest 129(9):3909–3923. https://doi.org/10.1172/JCI125332
Ma X, Xue X, Zhang J, Liang S, Xu C, Wang Y, Zhu J (2021) Liver expressed antimicrobial peptide 2 is associated with steatosis in mice and humans. Exp Clin Endocrinol Diabetes 129(8):601–610. https://doi.org/10.1055/a-1210-2357
Bhargava R, Luur S, Rodriguez Flores M, Emini M, Prechtl CG, Goldstone AP (2023) Postprandial increases in liver-gut hormone LEAP2 correlate with attenuated eating behavior in adults without obesity. Journal of the Endocrine Society 7(7):bvad061. https://doi.org/10.1210/jendso/bvad061
Byberg S, Blond MB, Holm S, Amadid H, Nielsen LB, Clemmensen KKB, Faerch K, Holst B (2023) LEAP2 is associated with cardiometabolic markers but is unchanged by antidiabetic treatment in people with prediabetes. Am J Physiol Endocrinol Metab 325(3):E244–E251. https://doi.org/10.1152/ajpendo.00023.2023
Hagemann CA, Zhang C, Hansen HH, Jorsal T, Rigbolt KTG, Madsen MR, Bergmann NC, Heimburger SMN, Falkenhahn M, Theis S, Breitschopf K, Holm S, Hedegaard MA, Christensen MB, Vilsboll T, Holst B, Vrang N, Jelsing J, Knop FK (2021) Identification and metabolic profiling of a novel human gut-derived LEAP2 fragment. J Clin Endocrinol Metab 106(2):e966–e981. https://doi.org/10.1210/clinem/dgaa803
Fittipaldi AS, Hernandez J, Castrogiovanni D, Lufrano D, De Francesco PN, Garrido V, Vitaux P, Fasano MV, Fehrentz JA, Fernandez A, Andreoli MF, Perello M (2020) Plasma levels of ghrelin, des-acyl ghrelin and LEAP2 in children with obesity: correlation with age and insulin resistance. Eur J Endocrinol 182(2):165–175. https://doi.org/10.1530/EJE-19-0684
Barja-Fernandez S, Lugilde J, Castelao C, Vazquez-Cobela R, Seoane LM, Dieguez C, Leis R, Tovar S (2021) Circulating LEAP-2 is associated with puberty in girls. Int J Obes (Lond) 45(3):502–514. https://doi.org/10.1038/s41366-020-00703-3
Varimo T, Miettinen PJ, Vaaralahti K, Toppari J, Huopio H, Voutilainen R, Tenhola S, Hero M, Raivio T (2022) Circulating Liver-enriched antimicrobial peptide-2 decreases during male puberty. J Endocrine Soc 6(3):bvac013. https://doi.org/10.1210/jendso/bvac013
Li J, Huang P, Xiong J, Liang X, Li M, Ke H, Chen C, Han Y, Huang Y, Zhou Y, Luo Z, Feng D, Chen C (2022) Serum levels of ghrelin and LEAP2 in patients with type 2 diabetes mellitus: correlation with circulating glucose and lipids. Endocr Connect. https://doi.org/10.1530/EC-22-0012
Holm S, Husted AS, Skov LJ, Morville TH, Hagemann CA, Jorsal T, Dall M, Jakobsen A, Klein AB, Treebak JT, Knop FK, Schwartz TW, Clemmensen C, Holst B (2022) Beta-hydroxybutyrate suppresses hepatic production of the ghrelin receptor antagonist LEAP2. Endocrinology. https://doi.org/10.1210/endocr/bqac038
Fittipaldi AS, Castrogiovanni D, Lufrano D, Saenz C, De Francesco PN, Lalonde T, Luyt LG, Cantel S, Fehrentz JA, Andreoli MF, Perello M (2022) Ghrelin proteolysis increases in plasma of men, but not women, with obesity. Life Sci 313:121305. https://doi.org/10.1016/j.lfs.2022.121305
Obesity: preventing and managing the global epidemic. Report of a WHO consultation (2000). World Health Organization technical report series 894:i-xii, 1–253
Guo J, Brager DC, Hall KD (2018) Simulating long-term human weight-loss dynamics in response to calorie restriction. Am J Clin Nutr 107(4):558–565. https://doi.org/10.1093/ajcn/nqx080
Smeets AJ, Soenen S, Luscombe-Marsh ND, Ueland O, Westerterp-Plantenga MS (2008) Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. J Nutr 138(4):698–702. https://doi.org/10.1093/jn/138.4.698
Hosoda H, Doi K, Nagaya N, Okumura H, Nakagawa E, Enomoto M, Ono F, Kangawa K (2004) Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin Chem 50(6):1077–1080. https://doi.org/10.1373/clinchem.2003.025841
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/BF00280883
Mani BK, Puzziferri N, He Z, Rodriguez JA, Osborne-Lawrence S, Metzger NP, Chhina N, Gaylinn B, Thorner MO, Thomas EL, Bell JD, Williams KW, Goldstone AP, Zigman JM (2019) LEAP2 changes with body mass and food intake in humans and mice. J Clin Invest. https://doi.org/10.1172/jci125332
Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, Stratton R, Delargy H, King N, Blundell JE (2000) The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr 84(4):405–415. https://doi.org/10.1017/s0007114500001719
Flint A, Raben A, Blundell JE, Astrup A (2000) Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 24(1):38–48. https://doi.org/10.1038/sj.ijo.0801083
Stratton RJ, Stubbs RJ, Hughes D, King N, Blundell JE, Elia M (1998) Comparison of the traditional paper visual analogue scale questionnaire with an Apple Newton electronic appetite rating system (EARS) in free living subjects feeding ad libitum. Eur J Clin Nutr 52(10):737–741. https://doi.org/10.1038/sj.ejcn.1600636
Hill AJ, Magson LD, Blundell JE (1984) Hunger and palatability: tracking ratings of subjective experience before, during and after the consumption of preferred and less preferred food. Appetite 5(4):361–371. https://doi.org/10.1016/s0195-6663(84)80008-2
Basulto J, Roura À, Calbet D (2008) Valoración de las sensaciones de apetito, hambre y saciedad mediante la utilización de sustitutivos de comidas (barritas). Ensayo aleatorizado, abierto y cruzado. Revista Española de Nutrición Humana y Dietética 12(2):47–55
Hernandez-Morante JJ, Galindo-Munoz JS, Barbera-Ortega Mdel C (2016) Development and validation of a smartphone application to analyze subjective appetite variables. Nutr Hosp 33(2):126. https://doi.org/10.20960/nh.126
Perello M, Cabral A, Cornejo MP, De Francesco PN, Fernandez G, Uriarte M (2019) Brain accessibility delineates the central effects of circulating ghrelin. J Neuroendocrinol 31(7):e12677. https://doi.org/10.1111/jne.12677
Cummings DE (2006) Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 89(1):71–84. https://doi.org/10.1016/j.physbeh.2006.05.022
Erdmann J, Hebeisen Y, Lippl F, Wagenpfeil S, Schusdziarra V (2007) Food intake and plasma ghrelin response during potato-, rice- and pasta-rich test meals. Eur J Nutr 46(4):196–203. https://doi.org/10.1007/s00394-007-0649-8
Greenman Y, Golani N, Gilad S, Yaron M, Limor R, Stern N (2004) Ghrelin secretion is modulated in a nutrient- and gender-specific manner. Clin Endocrinol 60(3):382–388. https://doi.org/10.1111/j.1365-2265.2004.01993.x
Blom WA, Lluch A, Stafleu A, Vinoy S, Holst JJ, Schaafsma G, Hendriks HF (2006) Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr 83(2):211–220. https://doi.org/10.1093/ajcn/83.2.211
Bennett NR, Boyne MS, Cooper RS, Royal-Thomas TY, Bennett FI, Luke A, Wilks RJ, Forrester TE (2009) Impact of adiponectin and ghrelin on incident glucose intolerance and on weight change. Clin Endocrinol 70(3):408–414. https://doi.org/10.1111/j.1365-2265.2008.03344.x
Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML (2001) Circulating ghrelin levels are decreased in human obesity. Diabetes 50(4):707–709. https://doi.org/10.2337/diabetes.50.4.707
Purnell JQ, Weigle DS, Breen P, Cummings DE (2003) Ghrelin levels correlate with insulin levels, insulin resistance, and high-density lipoprotein cholesterol, but not with gender, menopausal status, or cortisol levels in humans. J Clin Endocrinol Metab 88(12):5747–5752. https://doi.org/10.1210/jc.2003-030513
Maffeis C, Surano MG, Cordioli S, Gasperotti S, Corradi M, Pinelli L (2010) A high-fat vs. a moderate-fat meal in obese boys: nutrient balance, appetite, and gastrointestinal hormone changes. Obesity (Silver Spring) 18(3):449–455. https://doi.org/10.1038/oby.2009.271
Gregersen NT, Moller BK, Raben A, Kristensen ST, Holm L, Flint A, Astrup A (2011) Determinants of appetite ratings: the role of age, gender, BMI, physical activity, smoking habits, and diet/weight concern. Food Nutr Res. https://doi.org/10.3402/fnr.v55i0.7028
Leone A, De Amicis R, Pellizzari M, Bertoli S, Ravella S, Battezzati A (2022) Appetite ratings and ghrelin concentrations in young adults after administration of a balanced meal. Does sex matter? Biol Sex Diff 13(1):25. https://doi.org/10.1186/s13293-022-00434-2
Brand-Miller JC, Stockmann K, Atkinson F, Petocz P, Denyer G (2009) Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: analysis of a database of more than 1,000 foods. Am J Clin Nutr 89(1):97–105. https://doi.org/10.3945/ajcn.2008.26354
Acknowledgements
We thank to all the participants of the study, to the deputy director of the Instituto Biológico (Dr. H. Giudicci), who kindly allowed the use of the facilities to perform the studies, and to Camila Saenz and Ana Luz Kruger for their technical assistance.
Funding
This work was supported by grants from Fondo para la Investigación Científica y Tecnológica (FONCyT, PICT2017-3196, PICT2019-3054 and PICT2020-3270 to MP; PICT2019-256 to MFA), CONICET (PUE105 to MP), The National Qatar Research Foundation (NPRP13S-0209-200315 to AMH, MP and SAD) and Fundación Florencio Fiorini (to MFA).
Author information
Authors and Affiliations
Contributions
Conceptualization, formal analysis, original draft preparation, supervision, overall responsibility for the project: MFA, MP. Methodology, validation, formal analysis, investigation, resources and data curation; ASF, DC, PNDF, SV, FH, CRT, IM, MVF, HBS, SAD, FA, AMH, MFA, MP. Funding acquisition, formal analysis, reviewing and editing, supervision: AMH, SAD, MFA, MP. All authors approved the submitted and published versions.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest. The sponsors had no role in the design of the study, the collection, analyses or interpretation of data, writing of the manuscript, or the decision to publish the results.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andreoli, M.F., Fittipaldi, A.S., Castrogiovanni, D. et al. Pre-prandial plasma liver-expressed antimicrobial peptide 2 (LEAP2) concentration in humans is inversely associated with hunger sensation in a ghrelin independent manner. Eur J Nutr 63, 751–762 (2024). https://doi.org/10.1007/s00394-023-03304-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03304-8