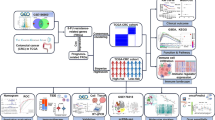
Abstract
Background
Wilm's tumor (WT) is one of the most common childhood urological tumors, ranking second in the incidence of pediatric abdominal tumors. The development of WT is associated with various factors, and the correlation with autophagy is currently unclear.
Purpose
To develop a new prognostic model of autophagy-related genes (ATG) for WT.
Methods
Using the Therapeutically applicable research to generate effective treatments (TARGET) database to screen for differentially expressed ATGs in WT and normal tissues. ATGs were screened for prognostic relevance to WT using one-way and multifactorial Cox regression analyses and prognostic models were constructed. The risk score was calculated according to the model, and the predictive ability of the constructed model was analyzed using the ROC (receiver operating characteristic) curve to verify the significance of the model for the prognosis of WT.
Results
Sixty-eight differentially expressed ATGs were identified by univariate Cox regression analysis, and two critical prognostic ATGs (CXCR4 and ERBB2) were identified by multivariate Cox regression analysis. Patients were divided into high-risk and low-risk groups according to the differential expression of these two ATGs. Kaplan–Meier (KM) curves showed a significant difference in survival time between the two groups. The critical prognostic ATGs were combined with race, age, and stage in a multifactorial regression analysis, and the final prognostic model was produced as a line graph.
Conclusion
The prognostic model of autophagy-related genes composed of the CXCR4 gene and ERBB2 gene has a specific predictive value for the prognosis of WT, and the present study provides a clear basis for future research on biomarkers and therapeutic targets.










Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Bozlu G, Citak EC (2018) Evaluation of renal tumors in children. Turk J Urol 44(3):268–273. https://doi.org/10.5152/tud.2018.70120
Guimei M, Eladl MA, Ranade AV et al (2019) Autophagy related markers (Beclin-1 and ATG4B) are strongly expressed in Wilms’ tumor and correlate with favorable histology. Histol Histopathol 34(1):47–56. https://doi.org/10.14670/hh-18-023
Ashford TP, Porter KR (1962) Cytoplasmic components in hepatic cell lysosomes. J Cell Bio 12(1):198–202. https://doi.org/10.1083/jcb.12.1.198
Li YJ, Lei YH, Yao N et al (2017) Autophagy and multidrug resistance in cancer. Chin J Cancer 36(1):52. https://doi.org/10.1186/s40880-017-0219-2
Izdebska M, Zielińska W, Hałas-Wiśniewska M et al (2019) Involvement of actin in autophagy and autophagy-dependent multidrug resistance in cancer. Cancers 11(8):1209. https://doi.org/10.3390/cancers11081209
Antunes F, Erustes AG, Costa AJ et al (2018) Autophagy and intermittent fasting: the connection for cancer therapy? Clinics (Sao Paulo, Brazil) 73(suppl 1):e814s. https://doi.org/10.6061/clinics/2018/e814s
Leri M, Scuto M, Ontario ML et al (2020) Healthy effects of plant polyphenols: molecular mechanisms. Int J Mol Sci 21(4):1250. https://doi.org/10.3390/ijms21041250
Qu X, Yu J, Bhagat G et al (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 112(12):1809–1820. https://doi.org/10.1172/jci20039
White E, DiPaola RS (2009) The double-edged sword of autophagy modulation in cancer. Clin Cancer Res 15(17):5308–5316. https://doi.org/10.1158/1078-0432.Ccr-07-5023
Butera G, Mullappilly N, Masetto F et al (2019) Regulation of autophagy by nuclear GAPDH and its aggregates in cancer and neurodegenerative disorders. Int J Mol Sci 20(9):2062. https://doi.org/10.3390/ijms20092062
Joffre C, Djavaheri-Mergny M, Pattingre S, Giuriato S (2017) L’autophagie: le yin et le yang des cancers [The yin and the yang of autophagy in cancer cells]. Med Sci (Paris) 33(3):328–334. https://doi.org/10.1051/medsci/20173303021
Jin S (2006) Autophagy, mitochondrial quality control, and oncogenesis. Autophagy 2(2):80–84. https://doi.org/10.4161/auto.2.2.2460
Yun CW, Lee SH (2018) The roles of autophagy in cancer. Int J Mol Sci. https://doi.org/10.3390/ijms19113466
Li X, Fan Z (2010) The epidermal growth factor receptor antibody cetuximab induces autophagy in cancer cells by downregulating HIF-1alpha and Bcl-2 and activating the beclin 1/hVps34 complex. Cancer Res 70(14):5942–5952. https://doi.org/10.1158/0008-5472.CAN-10-0157
Sridhar S, Botbol Y, Macian F et al (2012) Autophagy and disease: always two sides to a problem. J Pathol 226(2):255–273. https://doi.org/10.1002/path.3025
Bertout JA, Patel SA, Simon MC (2008) The impact of O2 availability on human cancer. Nat Rev Cancer 8(12):967–975. https://doi.org/10.1038/nrc2540
Wang J, Wang J, Sun Y, Song W, Nor JE, Wang CY, Taichman RS (2005) Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal 17(12):1578–1592. https://doi.org/10.1016/j.cellsig.2005.03.022. (Retraction in: Cell Signal. 2021Apr; 80: 109909)
Feng Y, Broder CC, Kennedy PE et al (1996) HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane G protein-coupled receptor. Science 272(5263):872–877. https://doi.org/10.1126/science.272.5263.872
Burger JA, Burger M, Kipps TJ (1999) Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood 94(11):3658–3667
Müller A, Homey B, Soto H et al (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410(6824):50–56. https://doi.org/10.1038/35065016
Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E, Peled A (2004) Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J 18(11):1240–1242. https://doi.org/10.1096/fj.03-0935fje
Furusato B, Mohamed A, Uhlén M et al (2010) CXCR4 and cancer. Pathol Int 60(7):497–505. https://doi.org/10.1111/j.1440-1827.2010.02548.x
Vandercappellen J, Van Damme J, Struyf S (2008) The role of CXC chemokines and their receptors in cancer. Cancer Lett 267(2):226–244. https://doi.org/10.1016/j.canlet.2008.04.050
Eck SM, Côté AL, Winkelman WD, Brinckerhoff CE (2009) CXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Mol Cancer Res 7(7):1033–1044. https://doi.org/10.1158/1541-7786.Mcr-09-0015
Kojima Y, Acar A, Eaton EN et al (2010) Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A 107(46):20009–20014. https://doi.org/10.1073/pnas.1013805107
Klein S, Abraham M, Bulvik B, Dery E, Weiss ID, Barashi N, Abramovitch R, Wald H, Harel Y, Olam D, Weiss L, Beider K, Eizenberg O, Wald O, Galun E, Pereg Y, Peled A (2018) CXCR4 promotes neuroblastoma growth and therapeutic resistance through miR-15a/16-1-mediated ERK and BCL2/Cyclin D1 pathways. Cancer Res 78(6):1471–1483. https://doi.org/10.1158/0008-5472
Peitzsch C, Kurth I, Kunz-Schughart L et al (2013) Discovery of the cancer stem cell related determinants of radioresistance. Radiother Oncol 108(3):378–387. https://doi.org/10.1016/j.radonc.2013.06.003
Bazley LA, Gullick WJ (2005) The epidermal growth factor receptor family. Endocr Relat Cancer 12(Suppl 1):S17-27. https://doi.org/10.1677/erc.1.01032
Holbro T, Hynes NE (2004) ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol 44:195–217. https://doi.org/10.1146/annurev.pharmtox.44.101802.121440
King CR, Kraus MH, Aaronson SA (1985) Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science 229(4717):974–976. https://doi.org/10.1126/science.2992089
Slamon DJ, Clark GM, Wong SG et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182. https://doi.org/10.1126/science.3798106
He C, Bian XY, Ni XZ et al (2013) Correlation of human epidermal growth factor receptor 2 expression with clinicopathological characteristics and prognosis in gastric cancer. World J Gastroenterol 19(14):2171–2178. https://doi.org/10.3748/wjg.v19.i14.2171
Baykara M, Benekli M, Ekinci O et al (2015) Clinical significance of HER2 overexpression in gastric and gastroesophageal junction cancers. J Gastrointest Surg 19(9):1565–1571. https://doi.org/10.1007/s11605-015-2888-y
Kurokawa Y, Matsuura N, Kimura Y et al (2015) Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer 18(4):691–697. https://doi.org/10.1007/s10120-014-0430-7
Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW, Heiss MM (2000) c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol 18(11):2201–2209. https://doi.org/10.1200/JCO.2000.18.11.2201
Wang S, Zheng G, Chen L et al (2011) Effect of HER-2/neu over-expression on prognosis in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev 12(6):1417–1423
Park DI, Yun JW, Park JH et al (2006) HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci 51(8):1371–1379. https://doi.org/10.1007/s10620-005-9057-1
Buza N (2021) HER2 testing in endometrial serous carcinoma: time for standardised pathology practice to meet the clinical demand. Arch Pathol Lab Med 145(6):687–691. https://doi.org/10.5858/arpa.2020-0207-RA
Author information
Authors and Affiliations
Contributions
HYS: Designed the study; analyzed the data; drafted the initial manuscript; and reviewed and revised the manuscript. MZ: Designed the study; reviewed and revised the manuscript; critically revised the manuscript YBZ: reviewed and revised the manuscript; critically revised the manuscript All authors approved the final manuscript as submitted and agree to be accountable for all aspects of work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, H., Zhang, M. & Zhang, Y. Construction of a prognostic model for autophagy in Wilm's tumor. Pediatr Surg Int 40, 122 (2024). https://doi.org/10.1007/s00383-024-05712-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-024-05712-1