Abstract
Purpose
Neonatal sepsis is a systemic inflammatory infection common in premature infants and a leading cause of mortality. Argon is an emerging interest in the field of noble gas therapy. Neonates with severe sepsis are frequently mechanically ventilated creating an opportunity for inhalation therapy. We aimed to investigate argon inhalation as a novel experimental therapy in neonatal sepsis.
Methods
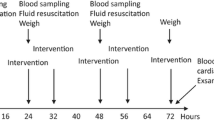
Sepsis was established in C57BL/6 neonatal mice by a lipopolysaccharide intraperitoneal injection on postnatal day 9. Septic pup mice were exposed to room air as well as non-septic controls. In the argon group, septic pup mice were exposed to argon (70% Ar, 30% O2) for 6 h in a temperature-controlled environment.
Results
At 6 h, survival was significantly enhanced when septic mice received argon compared to septic controls. Serum profiles of cytokine release were significantly attenuated as well as lung architecture restored.
Conclusions
Our findings suggest that argon inhalation as a novel treatment for neonatal sepsis, reducing mortality and counteracting the acute systemic inflammatory response in the blood and preserving the architecture of the lung. This research can contribute to a paradigm shift in the treatment and outcome of neonates with sepsis.




Similar content being viewed by others
Data availability
The data that support the findings in this study are not openly available but are available upon request from the corresponding author.
References
Glaser MA, Hughes LM, Jnah A, Newberry D (2021) Neonatal sepsis. Adv Neonatal Care 21:49–60. https://doi.org/10.1097/ANC.0000000000000769
Celik IH, Hanna M, Canpolat FE, Pammi M (2022) Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res 91:337–350. https://doi.org/10.1038/s41390-021-01696-z
Wynn JL, Wong HR (2017) Pathophysiology of neonatal sepsis. Fetal neonatal physiol. Elsevier, Amsterdam, pp 1536-1552e10. https://doi.org/10.1016/B978-0-323-35214-7.00152-9
Wynn JL (2016) Defining neonatal sepsis. Curr Opin Pediatr 28:135–140. https://doi.org/10.1097/MOP.0000000000000315
Greenfield KG, Badovinac VP, Griffith TS, Knoop KA (2021) Sepsis, cytokine storms, and immunopathology: the divide between neonates and adults. ImmunoHorizons 5:512–522. https://doi.org/10.4049/immunohorizons.2000104
Pietrasanta C, Pugni L, Ronchi A, Bottino I, Ghirardi B, Sanchez-Schmitz G et al (2019) Vascular endothelium in neonatal sepsis: basic mechanisms and translational opportunities. Front Pediatr 7:340. https://doi.org/10.3389/fped.2019.00340
Yadav P, Kumar Yadav S (2022) Progress in diagnosis and treatment of neonatal sepsis: a review article. J Nepal Med Assoc 60:318–324. https://doi.org/10.31729/jnma.7324
Cai S, Thompson DK, Anderson PJ, Yang JY-M (2019) Short- and long-term neurodevelopmental outcomes of very preterm infants with neonatal sepsis: a systematic review and meta-analysis. Children 6:131–131. https://doi.org/10.3390/children6120131
Spaggiari S, Kepp O, Rello-Varona S, Chaba K, Adjemian S, Pype J et al (2013) Antiapoptotic activity of argon and xenon. Cell Cycle 12:2636–2642. https://doi.org/10.4161/cc.25650
Rueckoldt H, Vangerow B, Marx G, Haubitz B, Cobas Meyer M, Piepenbrock S et al (1999) Xenon inhalation increases airway pressure in ventilated patients. Acta Anaesthesiol Scand 43:1060–1064. https://doi.org/10.1034/j.1399-6576.1999.431016.x
Le Nogue D, Lavaur J, Milet A, Ramirez-Gil JF, Katz I, Lemaire M et al (2020) Neuroprotection of dopamine neurons by xenon against low-level excitotoxic insults is not reproduced by other noble gases. J Neural Transm 127:27–34. https://doi.org/10.1007/s00702-019-02112-x
Liang M, Ahmad F, Dickinson R (2022) Neuroprotection by the noble gases argon and xenon as treatments for acquired brain injury: a preclinical systematic review and meta-analysis. Br J Anaesth 129:200–218. https://doi.org/10.1016/j.bja.2022.04.016
Nespoli F, Redaelli S, Ruggeri L, Fumagalli F, Olivari D, Ristagno G (2019) A complete review of preclinical and clinical uses of the noble gas argon: evidence of safety and protection. Ann Card Anaesth 22:122–122. https://doi.org/10.4103/aca.ACA_111_18
de Roux Q, Lidouren F, Kudela A, Slassi L, Kohlhauer M, Boissady E et al (2021) Argon attenuates multiorgan failure in relation with HMGB1 inhibition. Int J Mol Sci 22:3257–3257. https://doi.org/10.3390/ijms22063257
Ulbrich F, Lerach T, Biermann J, Kaufmann KB, Lagreze WA, Buerkle H et al (2016) Argon mediates protection by interleukin-8 suppression via a TLR2/TLR4/STAT3/NF-κB pathway in a model of apoptosis in neuroblastoma cells in vitro and following ischemia–reperfusion injury in rat retina in vivo. J Neurochem 138:859–873. https://doi.org/10.1111/jnc.13662
Alaedeen DI, Walsh MC, Chwals WJ (2006) Total parenteral nutrition–associated hyperglycemia correlates with prolonged mechanical ventilation and hospital stay in septic infants. J Pediatr Surg 41:239–244. https://doi.org/10.1016/j.jpedsurg.2005.10.045
Höllig A, Schug A, Fahlenkamp A, Rossaint R, Coburn M (2014) Argon Organo-Protective Network (AON). Argon: systematic review on neuro- and organoprotective properties of an “inert” gas. Int J Mol Sci 15:18175–18196. https://doi.org/10.3390/ijms151018175
Brites D, Fernandes A (2015) Bilirubin-induced neural impairment: a special focus on myelination, age-related windows of susceptibility and associated co-morbidities. Semin Fetal Neonatal Med 20:14–19. https://doi.org/10.1016/j.siny.2014.12.002
Limjunyawong N, Mock J, Mitzner W (2015) Instillation and fixation methods useful in mouse lung cancer research. J Vis Exp. https://doi.org/10.3791/52964
Crowley G, Kwon S, Caraher EJ, Haider SH, Lam R, Batra P et al (2019) Quantitative lung morphology: semi-automated measurement of mean linear intercept. BMC Pulm Med 19:206–206. https://doi.org/10.1186/s12890-019-0915-6
Barnette BW, Schumacher BT, Armenta RF, Wynn JL, Richardson A, Bradley JS et al (2021) Contribution of concurrent comorbidities to sepsis-related mortality in preterm infants ≤32 weeks of gestation at an academic neonatal intensive care network. Am J Perinatol. https://doi.org/10.1055/a-1675-2899
Aziz M, Jacob A, Yang W-L, Matsuda A, Wang P (2012) Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol 93:329–342. https://doi.org/10.1189/jlb.0912437
Tewabe T, Mohammed S, Tilahun Y, Melaku B, Fenta M, Dagnaw T et al (2017) Clinical outcome and risk factors of neonatal sepsis among neonates in Felege Hiwot referral Hospital, Bahir Dar, Amhara Regional State, North West Ethiopia 2016: a retrospective chart review. BMC Res Notes 10:265–265. https://doi.org/10.1186/s13104-017-2573-1
Bolognese AC, Yang W-L, Hansen LW, Denning N-L, Nicastro JM, Coppa GF et al (2018) Inhibition of necroptosis attenuates lung injury and improves survival in neonatal sepsis. Surgery 164:110–116. https://doi.org/10.1016/j.surg.2018.02.017
Martens A, Montoli M, Faggi G, Katz I, Pype J, Vanaudenaerde BM et al (2016) Argon and xenon ventilation during prolonged ex vivo lung perfusion. J Surg Res 201:44–52. https://doi.org/10.1016/j.jss.2015.10.007
Benjamin JT, Moore DJ, Bennett C, van der Meer R, Royce A, Loveland R et al (2018) Cutting edge: IL-1α and Not IL-1β drives IL-1R1-dependent neonatal murine sepsis lethality. J Immunol 201:2873–2878. https://doi.org/10.4049/jimmunol.1801089
Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS et al (2013) Role of cytokines as a double-edged sword in sepsis. Vivo Athens Greece 27:669–684
Jansen PM, de Jong IW, Hart M, Kim KJ, Aarden LA, Hinshaw LB et al (1996) Release of leukemia inhibitory factor in primate sepsis. Analysis of the role of TNF-alpha. J Immunol 156:4401–4407. https://doi.org/10.4049/jimmunol.156.11.4401
Remick DG (2007) Pathophysiology of sepsis. Am J Pathol 170:1435–1444. https://doi.org/10.2353/ajpath.2007.060872
Khaertynov KhS, Boichuk SV, Khaiboullina SF, Anokhin VA, Andreeva AA, Lombardi VC et al (2017) Comparative assessment of cytokine pattern in early and late onset of neonatal sepsis. J Immunol Res 2017:1–8. https://doi.org/10.1155/2017/8601063
Dinarello CA (2018) Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 281:8–27. https://doi.org/10.1111/imr.12621
Pisarek W, Jongh RD, Van Hoestenbergh R, Kenis G, Bosmans E, Heylen R (1997) Leukemia inhibitory factor during sepsis in adults and neonates. Crit Care 1:P015. https://doi.org/10.1186/cc21
Surbatovic M, Filipovic N, Radakovic S, Stankovic N, Slavkovic Z (2007) Immune cytokine response in combat casualties: blast or explosive trauma with or without secondary sepsis. Mil Med 172:190–195. https://doi.org/10.7205/MILMED.172.2.190
Riedemann NC, Guo R-F, Ward PA (2003) Novel strategies for the treatment of sepsis. Nat Med 9:517–524. https://doi.org/10.1038/nm0503-517
Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T et al (2008) Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112:1750–1758. https://doi.org/10.1182/blood-2008-01-130500
Bauer M (2022) The liver-gut-axis: initiator and responder to sepsis. Curr Opin Crit Care 28:216–220. https://doi.org/10.1097/MCC.0000000000000921
Sun J, Zhang J, Wang X, Ji F, Ronco C, Tian J et al (2020) Gut-liver crosstalk in sepsis-induced liver injury. Crit Care 24:614–614. https://doi.org/10.1186/s13054-020-03327-1
Li Q, Zhang Q, Wang C, Liu X, Li N, Li J (2009) Disruption of tight junctions during polymicrobial sepsis in vivo. J Pathol 218:210–221. https://doi.org/10.1002/path.2525
Cardoso FL, Herz J, Fernandes A, Rocha J, Sepodes B, Brito MA et al (2015) Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J Neuroinflammation 12:82. https://doi.org/10.1186/s12974-015-0299-3
Nowrangi DS, Tang J, Zhang JH (2014) Argon gas: a potential neuroprotectant and promising medical therapy. Med Gas Res 4:3–3. https://doi.org/10.1186/2045-9912-4-3
Mercier J-C, Hummler H, Durrmeyer X, Sanchez-Luna M, Carnielli V, Field D et al (2010) Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. The Lancet 376:346–354. https://doi.org/10.1016/S0140-6736(10)60664-2
Acknowledgements
We would like to thank Rebeca Figueira for assisting in the processing and analysis of lung tissue.
Funding
AP is supported by a Canadian Institutes of Health Research (CIHR) Foundation Grant 353857, Project Grant 487457, and Project Grant 496719.
Author information
Authors and Affiliations
Contributions
FB: conception and design, collection and/or assembly of data, data analysis and interpretation. SC, DL, GB, CL, BL, JM contributed to the research, discussion, writing, and editing of this manuscript. AP: conception and design, financial support, final approval of manuscript. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balsamo, F., Li, B., Chusilp, S. et al. Argon inhalation attenuates systemic inflammation and rescues lung architecture during experimental neonatal sepsis. Pediatr Surg Int 40, 21 (2024). https://doi.org/10.1007/s00383-023-05596-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05596-7