Abstract
Purpose
Congenital diaphragmatic hernia (CDH) survivors may experience neurodevelopmental impairment, whose etiology remains elusive. Preclinical evidence indicates that amniotic fluid stem cell extracellular vesicle (AFSC-EV) administration promotes lung development but their effects on other organs are unknown. Herein, we investigated the brain of rat fetuses with CDH for signs of inflammation and response to AFSC-EVs.
Methods
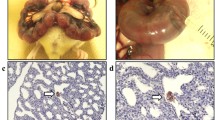
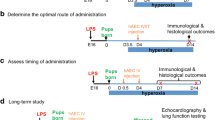
CDH was induced by maternal nitrofen administration at E9.5. At E18.5, fetuses were injected intra-amniotically with saline or AFSC-EVs (isolated by ultracentrifugation, characterized as per MISEV guidelines). Fetuses from vehicle-gavaged dams served as controls. Groups were compared for: lung hypoplasia, TNFa and IL-1B brain expression, and activated microglia (Iba1) density in the subgranular zone (SGZ).
Results
CDH lungs had fewer airspaces compared to controls, whereas AFSC-EV-treated lungs had rescued branching morphogenesis. Fluorescently labeled AFSC-EVs injected intra-amniotically into CDH fetuses had fluorescent signal in the brain. Compared to controls, the brain of CDH fetuses had higher TNFa and IL-1B levels, and increased activated microglia density. Conversely, the brain of AFSC-EV treated fetuses had inflammatory marker expression levels and microglia density similar to controls.
Conclusion
This study shows that the brain of rat fetuses with CDH has signs of inflammation that are abated by the intra-amniotic administration of AFSC-EVs.



Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper.
References
Zani A, Chung WK, Deprest J, Harting MT, Jancelewicz T, Kunisaki SM, Patel N, Antounians L, Puligandla PS, Keijzer R (2022) Congenital diaphragmatic hernia. Nat Rev Dis Primers 8(1):37. https://doi.org/10.1038/s41572-022-00362-w
Montalva L, Raffler G, Riccio A, Lauriti G, Zani A (2020) Neurodevelopmental impairment in children with congenital diaphragmatic hernia: Not an uncommon complication for survivors. J Pediatr Surg 55(4):625–634. https://doi.org/10.1016/j.jpedsurg.2019.05.021
Van der Veeken L, Russo FM, Litwinska E, Gomez O, Emam D, Lewi L, Basurto D, Van der Veeken S, De Catte L, Gratacos E, Eixarch E, Nicolaides K, Deprest J (2022) Prenatal cerebellar growth is altered in congenital diaphragmatic hernia on ultrasound. Prenat Diagn 42(3):330–337. https://doi.org/10.1002/pd.5993
Kosiv KA, Moon-Grady A, Hogan W, Keller R, Rapoport R, Rogers E, Feldstein VA, Lee H, Peyvandi S (2021) Fetal cerebrovascular impedance is reduced in left congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 57(3):386–391. https://doi.org/10.1002/uog.21992
Machado-Rivas F, Choi JJ, Alejandra Bedoya M, Acosta Buitrago L, Velasco-Annis C, Afacan O, Barnewolt C, Estroff J, Warfield SK, Gholipour A, Jaimes C (2023) Brain growth in fetuses with congenital diaphragmatic hernia. J Neuroimaging 33(4):617–624. https://doi.org/10.1111/jon.13096
Radhakrishnan R, Merhar SL, Burns P, Zhang B, Lim FY, Kline-Fath BM (2019) Fetal brain morphometry on prenatal magnetic resonance imaging in congenital diaphragmatic hernia. Pediatr Radiol 49(2):217–223. https://doi.org/10.1007/s00247-018-4272-z
Emam D, Aertsen M, Van der Veeken L, Fidon L, Patkee P, Kyriakopoulou V, De Catte L, Russo F, Demaerel P, Vercauteren T, Rutherford M, Deprest J (2023) Longitudinal MRI evaluation of brain development in fetuses with congenital diaphragmatic hernia around the time of fetal endotracheal occlusion. AJNR Am J Neuroradiol 44(2):205–211. https://doi.org/10.3174/ajnr.A7760
Johng S, Licht DJ, Hedrick HL, Rintoul N, Linn RL, Gebb JS, Xiao R, Massey SL (2023) Prenatal brain maturation is delayed in neonates with congenital diaphragmatic hernia. J Pediatr 264:113738. https://doi.org/10.1016/j.jpeds.2023.113738
Biouss G, Antounians A, Aguet J, Kopcalic K, Fakhari N, Baranger J, Mertens L, Villemain O, Zani A (2023) Unveiling fetal brain changes in congenital diaphragmatic hernia: hypoxic injury with loss of progenitor cells, neurons and oligodendrocytes. BioRXiv. https://doi.org/10.1101/2023.09.23.559137
Wagner R, Amonkar GM, Wang W, Shui JE, Bankoti K, Tse WH, High FA, Zalieckas JM, Buchmiller TL, Zani A, Keijzer R, Donahoe PK, Lerou PH, Ai X (2023) A tracheal aspirate-derived airway basal cell model reveals a proinflammatory epithelial defect in congenital diaphragmatic hernia. Am J Respir Crit Care Med 207(9):1214–1226. https://doi.org/10.1164/rccm.202205-0953OC
Varisco BM (2023) Nuclear factor-κB keeps basal cells undifferentiated in congenital diaphragmatic hernia. Am J Respir Crit Care Med 207(9):1122–1123. https://doi.org/10.1164/rccm.202302-0290ED
Shima H, Ohshiro K, Taira Y, Miyazaki E, Oue T, Puri P (1999) Antenatal dexamethasone suppresses tumor necrosis factor-alpha expression in hypoplastic lung in nitrofen-induced diaphragmatic hernia in rats. Pediatr Res 46(5):633–637. https://doi.org/10.1203/00006450-199911000-00023
Schaible T, Reineke J, Gortner L, Monz D, Schaffelder R, Tutdibi E (2017) Are cytokines useful biomarkers to determine disease severity in neonates with congenital diaphragmatic hernia? Am J Perinatol 34(7):648–654. https://doi.org/10.1055/s-0036-1597133
Herrera-Rivero M, Zhang R, Heilmann-Heimbach S, Mueller A, Bagci S, Dresbach T, Schröder L, Holdenrieder S, Reutter HM, Kipfmueller F (2018) Circulating microRNAs are associated with pulmonary hypertension and development of chronic lung disease in congenital diaphragmatic hernia. Sci Rep 8(1):10735. https://doi.org/10.1038/s41598-018-29153-8
Perry R, Stein J, Young G, Ramanathan R, Seri I, Klee L, Friedlich P (2013) Antithrombin III administration in neonates with congenital diaphragmatic hernia during the first three days of extracorporeal membrane oxygenation. J Pediatr Surg 48(9):1837–1842. https://doi.org/10.1016/j.jpedsurg.2012.11.037
Pavcnik-Arnol M, Bonac B, Groselj-Grenc M, Derganc M (2010) Changes in serum procalcitonin, interleukin 6, interleukin 8 and C-reactive protein in neonates after surgery. Eur J Pediatr Surg 20(4):262–266. https://doi.org/10.1055/s-0030-1253358
Fleck S, Bautista G, Keating SM, Lee TH, Keller RL, Moon-Grady AJ, Gonzales K, Norris PJ, Busch MP, Kim CJ, Romero R, Lee H, Miniati D, MacKenzie TC (2013) Fetal production of growth factors and inflammatory mediators predicts pulmonary hypertension in congenital diaphragmatic hernia. Pediatr Res 74(3):290–298. https://doi.org/10.1038/pr.2013.98
Okawada M, Kobayashi H, Tei E, Okazaki T, Lane GJ, Yamataka A (2007) Serum monocyte chemotactic protein-1 levels in congenital diaphragmatic hernia. Pediatr Surg Int 23(5):487–491. https://doi.org/10.1007/s00383-006-1858-6
Antounians L, Figueira RL, Kukreja B, Zani-Ruttenstock E, Khalaj K, Montalva L, Doktor F, Obed M, Blundell M, Wu T, Chan C, Wagner W, Lacher M, Wilson MD, Kalish BT, Zani A (2022) Administration of amniotic fluid stem cell extracellular vesicles promotes development of fetal hypoplastic lungs by immunomodulating lung macrophages. BioRXiv. https://doi.org/10.1101/2022.11.29.518388
Antounians L, Catania VD, Montalva L, Liu BD, Hou H, Chan C, Matei AC, Tzanetakis A, Li B, Figueira RL, da Costa KM, Wong AP, Mitchell R, David AL, Patel K, De Coppi P, Sbragia L, Wilson MD, Rossant J, Zani A (2021) Fetal lung underdevelopment is rescued by administration of amniotic fluid stem cell extracellular vesicles in rodents. Sci Transl Med 13:5941. https://doi.org/10.1126/scitranslmed.aax5941
Khalaj K, Antounians L, Figueira RL, Post M, Zani A (2022) Autophagy is impaired in fetal hypoplastic lungs and rescued by administration of amniotic fluid stem cell extracellular vesicles. Am J Respir Crit Care Med 206(4):476–487. https://doi.org/10.1164/rccm.202109-2168OC
Khalaj K, Figueira RL, Antounians L, Gandhi S, Wales M, Montalva L, Biouss G, Zani A (2022) Treatment with amniotic fluid stem cell extracellular vesicles promotes fetal lung branching and cell differentiation at canalicular and saccular stages in experimental pulmonary hypoplasia secondary to congenital diaphragmatic hernia. Stem Cells Transl Med 11(10):1089–1102. https://doi.org/10.1093/stcltm/szac063
Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 14(4):27066. https://doi.org/10.3402/jev.v4.27066
Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C (2014) Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 22(3):26913. https://doi.org/10.3402/jev.v3.26913
Antounians L, Tzanetakis A, Pellerito O, Catania VD, Sulistyo A, Montalva L, McVey MJ, Zani A (2019) The regenerative potential of amniotic fluid stem cell extracellular vesicles: lessons learned by comparing different isolation techniques. Sci Rep 9(1):1837. https://doi.org/10.1038/s41598-018-38320-w
Kluth D, Kangah R, Reich P, Tenbrinck R, Tibboel D, Lambrecht W (1990) Nitrofen-induced diaphragmatic hernias in rats: an animal model. J Pediatr Surg 25(8):850–854. https://doi.org/10.1016/0022-3468(90)90190-k
Montalva L, Zani A (2019) Assessment of the nitrofen model of congenital diaphragmatic hernia and of the dysregulated factors involved in pulmonary hypoplasia. Pediatr Surg Int 35(1):41–61. https://doi.org/10.1007/s00383-018-4375-5
Iritani I (1984) Experimental study on embryogenesis of congenital diaphragmatic hernia. Anat Embryol (Berl) 169(2):133–139. https://doi.org/10.1007/BF00303142
Montalva L, Antounians L, Zani A (2019) Pulmonary hypertension secondary to congenital diaphragmatic hernia: factors and pathways involved in pulmonary vascular remodeling. Pediatr Res 85(6):754–768. https://doi.org/10.1038/s41390-019-0345-4
Hsia CC, Hyde DM, Ochs M, Weibel ER, ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure (2010) An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181(4):394–418. https://doi.org/10.1164/rccm.200809-1522ST
Crowley G, Kwon S, Caraher EJ, Haider SH, Lam R, Batra P, Melles D, Liu M, Nolan A (2019) Quantitative lung morphology: semi-automated measurement of mean linear intercept. BMC Pulm Med 19(1):206. https://doi.org/10.1186/s12890-019-0915-6
Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ (2013) Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107:1–16. https://doi.org/10.1016/j.pneurobio.2013.04.001
Kaur C, Rathnasamy G, Ling EA (2013) Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina. J Neuroimmune Pharmacol 8(1):66–78. https://doi.org/10.1007/s11481-012-9347-2
Kremsky I, Ma Q, Li B, Dasgupta C, Chen X, Ali S, Angeloni S, Wang C, Zhang L (2023) Fetal hypoxia results in sex- and cell type-specific alterations in neonatal transcription in rat oligodendrocyte precursor cells, microglia, neurons, and oligodendrocytes. Cell Biosci 13(1):58. https://doi.org/10.1186/s13578-023-01012-8
Kaur C, Sivakumar V, Ang LS, Sundaresan A (2006) Hypoxic damage to the periventricular white matter in neonatal brain: role of vascular endothelial growth factor, nitric oxide and excitotoxicity. J Neurochem 98(4):1200–1216. https://doi.org/10.1111/j.1471-4159.2006.03964.x
Woods RM, Lorusso JM, Fletcher J, ElTaher H, McEwan F, Harris I, Kowash HM, D’Souza SW, Harte M, Hager R, Glazier JD (2023) Maternal immune activation and role of placenta in the prenatal programming of neurodevelopmental disorders. Neuronal Signal 7(2):NS20220064. https://doi.org/10.1042/NS20220064
Mueller FS, Scarborough J, Schalbetter SM, Richetto J, Kim E, Couch A, Yee Y, Lerch JP, Vernon AC, Weber-Stadlbauer U, Meyer U (2021) Behavioral, neuroanatomical, and molecular correlates of resilience and susceptibility to maternal immune activation. Mol Psychiatry 26(2):396–410. https://doi.org/10.1038/s41380-020-00952-8
Shin Yim Y, Park A, Berrios J, Lafourcade M, Pascual LM, Soares N, Yeon Kim J, Kim S, Kim H, Waisman A, Littman DR, Wickersham IR, Harnett MT, Huh JR, Choi GB (2017) Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549(7673):482–487. https://doi.org/10.1038/nature23909
Ramos-Zaldívar HM, Polakovicova I, Salas-Huenuleo E, Corvalán AH, Kogan MJ, Yefi CP, Andia ME (2022) Extracellular vesicles through the blood-brain barrier: a review. Fluids Barriers CNS 19(1):60. https://doi.org/10.1186/s12987-022-00359-3
Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, Farhoodi HP, Zhang SX, Zimak J, Ségaliny A, Riazifar M, Pham V, Digman MA, Pone EJ, Zhao W (2016) Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng 9(4):509–529. https://doi.org/10.1007/s12195-016-0458-3
Matsumoto J, Stewart T, Sheng L, Li N, Bullock K, Song N, Shi M, Banks WA, Zhang J (2017) Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol Commun 5(1):71. https://doi.org/10.1186/s40478-017-0470-4
Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL (2020) Transport of extracellular vesicles across the blood-brain barrier: Brain pharmacokinetics and effects of inflammation. Int J Mol Sci 21(12):4407. https://doi.org/10.3390/ijms21124407
Abdelsalam M, Ahmed M, Osaid Z, Hamoudi R, Harati R (2023) Insights into exosome transport through the blood-brain barrier and the potential therapeutical applications in brain diseases. Pharmaceuticals (Basel) 16(4):571. https://doi.org/10.3390/ph16040571
Malhotra A, Castillo-Melendez M, Allison BJ, Sutherland AE, Nitsos I, Pham Y, McDonald CA, Fahey MC, Polglase GR, Jenkin G, Miller SL (2020) Neurovascular effects of umbilical cord blood-derived stem cells in growth-restricted newborn lambs: UCBCs for perinatal brain injury. Stem Cell Res Ther 11(1):17. https://doi.org/10.1186/s13287-019-1526-0
Davidson JO, van den Heuij LG, Fraser M, Wassink G, Miller SL, Lim R, Wallace EM, Jenkin G, Gunn AJ, Bennet L (2021) Window of opportunity for human amnion epithelial stem cells to attenuate astrogliosis after umbilical cord occlusion in preterm fetal sheep. Stem Cells Transl Med 10(3):427–440. https://doi.org/10.1002/sctm.20-0314
Chand K, Nano R, Wixey J, Patel J (2022) Stem cell therapy for neuroprotection in the growth-restricted newborn. Stem Cells Transl Med 11(4):372–382. https://doi.org/10.1093/stcltm/szac005
Gamage TKJB, Fraser M (2021) The role of extracellular vesicles in the developing brain: current perspective and promising source of biomarkers and therapy for perinatal brain injury. Front Neurosci 15:744840. https://doi.org/10.3389/fnins.2021.744840
Thomi G, Surbek D, Haesler V, Joerger-Messerli M, Schoeberlein A (2019) Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther 10(1):105. https://doi.org/10.1186/s13287-019-1207-z. (Erratum in: Stem Cell Res Ther (2022) 13(1):364)
Thomi G, Joerger-Messerli M, Haesler V, Muri L, Surbek D, Schoeberlein A (2019) Intranasally administered exosomes from umbilical cord stem cells have preventive neuroprotective effects and contribute to functional recovery after perinatal brain injury. Cells 8(8):855. https://doi.org/10.3390/cells8080855
Kaminski N, Köster C, Mouloud Y, Börger V, Felderhoff-Müser U, Bendix I, Giebel B, Herz J (2020) Mesenchymal stromal cell-derived extracellular vesicles reduce neuroinflammation, promote neural cell proliferation and improve oligodendrocyte maturation in neonatal hypoxic-ischemic brain injury. Front Cell Neurosci 14:601176. https://doi.org/10.3389/fncel.2020.601176
Sisa C, Kholia S, Naylor J, Herrera Sanchez MB, Bruno S, Deregibus MC, Camussi G, Inal JM, Lange S, Hristova M (2019) Mesenchymal stromal cell derived extracellular vesicles reduce hypoxia-ischaemia induced perinatal brain injury. Front Physiol 19(10):282. https://doi.org/10.3389/fphys.2019.00282
Xin D, Li T, Chu X, Ke H, Yu Z, Cao L, Bai X, Liu D, Wang Z (2020) Mesenchymal stromal cell-derived extracellular vesicles modulate microglia/macrophage polarization and protect the brain against hypoxia-ischemic injury in neonatal mice by targeting delivery of miR-21a-5p. Acta Biomater 113:597–613. https://doi.org/10.1016/j.actbio.2020.06.037
Chu X, Liu D, Li T, Ke H, Xin D, Wang S, Cao Y, Xue H, Wang Z (2020) Hydrogen sulfide-modified extracellular vesicles from mesenchymal stem cells for treatment of hypoxic-ischemic brain injury. J Control Release 328:13–27. https://doi.org/10.1016/j.jconrel.2020.08.037
Gussenhoven R, Klein L, Ophelders DRMG, Habets DHJ, Giebel B, Kramer BW, Schurgers LJ, Reutelingsperger CPM, Wolfs TGAM (2019) Annexin A1 as neuroprotective determinant for blood-brain barrier integrity in neonatal hypoxic-ischemic encephalopathy. J Clin Med 8(2):137. https://doi.org/10.3390/jcm8020137
Han J, Yang S, Hao X, Zhang B, Zhang H, Xin C, Hao Y (2021) Extracellular vesicle-derived microRNA-410 from mesenchymal stem cells protects against neonatal hypoxia-ischemia brain damage through an HDAC1-dependent EGR2/Bcl2 axis. Front Cell Dev Biol 8:579236. https://doi.org/10.3389/fcell.2020.579236
Ahn SY, Sung DK, Kim YE, Sung S, Chang YS, Park WS (2021) Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cell-derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats. Stem Cells Transl Med 10(3):374–384. https://doi.org/10.1002/sctm.20-0301
Drommelschmidt K, Serdar M, Bendix I, Herz J, Bertling F, Prager S, Keller M, Ludwig AK, Duhan V, Radtke S, de Miroschedji K, Horn PA, van de Looij Y, Giebel B, Felderhoff-Müser U (2017) Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun 60:220–232. https://doi.org/10.1016/j.bbi.2016.11.011
Ophelders DR, Wolfs TG, Jellema RK, Zwanenburg A, Andriessen P, Delhaas T, Ludwig AK, Radtke S, Peters V, Janssen L, Giebel B, Kramer BW (2016) Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem Cells Transl Med 5(6):754–763. https://doi.org/10.5966/sctm.2015-0197
Acknowledgements
The authors would like to thank Lindsey (Renji) Li, Miguel Garcia, Tasneem Islam, and Cindy Yu for their assistance in conducting some of the experiments, and the Imaging Facility and Lab Animal Services core facilities at the Hospital for Sick Children, Toronto, Canada. This project was supported by the Canadian Institutes of Health Research (CIHR) Project Grant (175300), SickKids Congenital Diaphragmatic Hernia Fund (R00DH00000), and Perioperative Services Summer Studentship Program at the Hospital for Sick Children, Toronto, Canada. Some of the equipment used in this study was supported by the 3D (Diet, Digestive Tract and Disease) Centre funded by the Canadian Foundation for Innovation and Ontario Research Fund, project number 19442 and 30961.
Author information
Authors and Affiliations
Contributions
Conceptualization: MB, LA, AZ. Methodology: MB, FD, RLF, LA, GB, KK, AZ. Visualization: MB, FD, RLF, LA, KK. Project administration: LA, AZ. Funding acquisition: LA, AZ. Supervision: RLF, FD, LA, AZ. Writing—original draft preparation: MB, LA, AZ. Writing—review and editing: MB, LA, AZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was obtained for all animal experiments from the Hospital for Sick Children, conducted under AUPs #49892 and #65210. All experiments were performed in accordance with the Canadian Council on Animal Care guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Blundell, M., Doktor, F., Figueira, R.L. et al. Anti-inflammatory effects of antenatal administration of stem cell derived extracellular vesicles in the brain of rat fetuses with congenital diaphragmatic hernia. Pediatr Surg Int 39, 291 (2023). https://doi.org/10.1007/s00383-023-05578-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05578-9