Abstract
Torpor is characterized by an extreme reduction in metabolism and a common energy-saving strategy of heterothermic animals. Torpor is often associated with cold temperatures, but in the last decades, more diverse and flexible forms of torpor have been described. For example, tropical bat species maintain a low metabolism and heart rate at high ambient and body temperatures. We investigated whether bats (Nyctalus noctula) from the cooler temperate European regions also show this form of torpor with metabolic inhibition at high body temperatures, and whether this would be as pronounced in reproductive as in non-reproductive bats. We simultaneously measured metabolic rate, heart rate, and skin temperature in non-reproductive and pregnant females at a range of ambient temperatures. We found that they can decouple metabolic rate and heart rate from body temperature: they maintained an extremely low metabolism and heart rate when exposed to ambient temperatures changing from 0 to 32.5 °C, irrespective of reproductive status. When we simulated natural temperature conditions, all non-reproductive bats used torpor throughout the experiment. Pregnant bats used variable strategies from torpor, to maintaining normothermy, or a combination of both. Even a short torpor bout during the day saved up to 33% of the bats' total energy expenditure. Especially at higher temperatures, heart rate was a much better predictor of metabolic rate than skin temperature. We suggest that the capability to flexibly save energy across a range of ambient temperatures within and between reproductive states may be an important ability of these bats and possibly other temperate-zone heterotherms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most organisms are energetically limited by resource availability. This has led to behavioral and physiological adaptations that involve using resources efficiently, optimizing metabolic processes, and minimizing energy expenditure. One of the most efficient strategies to minimize energy expenditure in heterothermic endotherms is torpor. Torpor is a controlled reduction of metabolic rate (Stawski et al. 2014) associated with a reduction in heart rate (Ruf and Geiser 2015; Geiser 2021). Most often, this reduced metabolism is associated with reduced body temperature when animals thermoconform to low ambient temperatures (Geiser 2021).
Torpor is commonly used by small heterothermic mammals from the temperate-zones such as ground squirrels, dormice, or bats (Geiser 2004). Selection for efficient energy-saving strategies is thought to be especially strong on temperate-zone bats due to the high costs of active flight (Schmidt-Nielsen 1970), and also because many species do not accumulate much body fat except in the pre-hibernation season (Kunz et al. 1998). The energy intake of bats is unpredictable as they often feed on ephemeral insect swarms that fluctuate in availability both in time and space (Ruczyński et al. 2020). To compensate for this, many bats select roosts with relatively low and stable ambient temperatures such as caves and tree cavities (Kunz 1982). This is thought to facilitate torpor enabling bats to reduce their body temperature and metabolism more than would be possible at higher ambient temperatures (Hamilton and Barclay 1994; Geiser 2004).
In contrast, tropical or desert bat species often experience high ambient day temperatures. This limits how far they can lower body temperature when using torpor during the day, but significant energetic savings at temperatures above the thermoneutral zone (TNZ) have also been found (Maloney et al. 1999; Bondarenco et al. 2014; Reher and Dausmann 2021). For example, the metabolism of Commerson's leaf-nosed bats (Macronycteris commersoni) is 80% lower at ambient temperatures of more than 41 °C compared to their resting metabolic rate (RMR; Reher and Dausmann 2021). In Pallas’ free-tailed bats (Molossus molossus) similar energy savings of 60% are associated with extremely low heart rates at high ambient and thus body temperatures (Dechmann et al. 2011; O'Mara et al. 2017a). These studies were only able to detect this state of low energy consumption at high ambient temperatures using direct measurements of metabolic rate or high-resolution data on heart rate (O'Mara et al. 2017a; Reher and Dausmann 2021), instead of pre-defined temperature thresholds (Audet and Fenton 1988; Barclay et al. 1996; Jonasson and Willis 2012).
Ambient temperatures can influence the depth and frequency of torpor bouts, but additional constraints on torpor use likely exist for both sexes during reproduction. In the temperate zone, sperm-producing males as well as pregnant and lactating females use torpor less frequently and in shorter and shallower bouts than non-reproductive individuals (Grinevitch et al. 1995; Lausen and Barclay 2003; Dzal and Brigham 2013; Komar et al. 2020). This is likely because extended torpor inhibits sperm development, embryonic growth, and milk production (Hamilton and Barclay 1994; Dietz and Kalko 2006; Adams 2010).
We aimed to determine if bats from temperate regions can also maintain a low metabolism and heart rate at high ambient temperatures to save energy during the reproductive and non-reproductive season. We then wanted to quantify how variation in torpor use translates into energy expenditure in reproductive and non-reproductive bats. We worked with reproductive and non-reproductive female common noctule bats (Nyctalus noctula). Nyctalus noctula is a 30 g insectivorous European bat that regularly uses torpor (Braun and Dieterlen 2003). Their short activity period with on average 2 h of foraging and the ephemerality of their insect prey likely increases the pressure to conserve energy, especially during energetically challenging reproduction. We exposed bats to varying ambient temperatures while simultaneously monitoring three physiological parameters: metabolic rate, heart rate, and skin temperature. We tested two hypotheses: (1) regardless of reproductive status, N. noctula are able to use torpor, reducing metabolism and heart rates at high ambient and thus body temperatures; and (2) under natural temperature conditions, reproductive individuals enter torpor less frequently and maintain higher metabolic and heart rates than non-reproductive individuals to enable fetal development. In addition, we quantified how variation in torpor use translates into energy expenditures in reproductive and non-reproductive N. noctula and re-examined previous results showing that heart rate is a better predictor of oxygen consumption than skin temperature.
Materials and methods
Study populations and bat capture
Female N. noctula migrate and are not present in the hibernation area during the reproductive season in summer (Dechmann et al. 2014, 2017; Lehnert et al. 2018). Thus, we conducted our study with individuals from two different populations. We worked with non-reproductive female N. noctula from the population in Southern Germany (hereafter “Konstanz”, 47°39′59.8′′, N 9°10′53.6′′ E) just after hibernation (post-hibernation; April 3rd–16th 2019; n = 10; mean body mass = 27.22 g) and just before hibernation (pre-hibernation; October 1st–17th 2019; n = 9; mean body mass = 33.14 g). We studied reproductive female bats from the population in the Białowieża Primeval Forest in Eastern Poland (hereafter “Białowieża”, 52°41′59.9′′ N, 23°52′04.5′′ E) during early pregnancy (May 18th–31st 2019; n = 12; mean body mass = 32.42 g). In Konstanz, we removed the bats from bat boxes during the day and transported them in individual soft cloth bags to a laboratory facility at the nearby Max Planck Institute of Animal Behavior. In Białowieża, we caught females emerging from a maternity colony at dusk with mist nets (Ecotone, Gdynia, Poland) and transported them to the nearby Mammal Research Institute of the Polish Academy of Sciences. All bats were adult at the time of capture.
After transport to the laboratory, we weighed bats with a digital scale (± 0.01 g; Kern & Sohn, Bahlingen, Germany). With each bat, we performed two respirometry experiments with different temperature regimes (see “Experimental design”). When not involved in an experiment, we kept single individuals in artificial roosts in hollow tree trunks (Ruczyński et al. 2007). Bats received mealworms and water ad libitum during their natural foraging time at dusk and we weighed them every day before and after each experiment. Bats were kept for a maximum of three days after which we released them into the box they had been removed from in Konstanz or at the capture site in Białowieża.
Experimental design
Bats were placed into the respirometry chambers between 21:00 and 23:30 the night prior to each experiment to acclimate. We then began experiments at 06:00 the following morning. In the first respirometry experiment (“6 h-experiment”), we tested our first hypothesis that regardless of reproductive status, N. noctula are able to use torpor, reducing metabolism and heart rates (fH) in spite of high ambient (Ta) and thus body temperatures (Tskin). We exposed bats to a range of temperatures in six increasing increments of Ta (0, 7.5, 15, 22.5, 27.5, 32.5 °C) for 1 h each (06:00–12:00). Ta was measured using iButtons (DS1922L, Maxim Integrated Products, San Jose, California, USA) inside the small plastic containers used for the respirometry experiments (see below). To address our second hypothesis, that under simulated natural temperature conditions reproductive individuals would use torpor less frequently and maintain higher metabolic rates than non-reproductive individuals, we conducted a second experiment (“12 h-experiment”). We averaged Ta from Białowieża and Konstanz from the previous 2 years for each month and calculated means for 4-h time periods during the day and a single mean temperature during the night (06:00–10:00, 10:00–14:00, 14:00–18:00, and 18:00–06:00). Resulting experimental Ta during non-reproduction were 9.1 °C, 13.6 °C, 15.0 °C and 9.0 °C (post-hibernation), and 9.1 °C, 12.2 °C, 12.8 °C and 9.2 °C (pre-hibernation) and during reproduction 13.4 °C, 17.5 °C, 18.6 °C and 12.7 °C (pregnancy).
Heart rate transmitter attachment and monitoring
We attached external heart rate transmitters (ca. 0.8 g, 5 × 3 × 8 mm; SP2000 HR Sparrow Systems, Fisher, Illinois, USA) to each bat (Dechmann et al. 2011; O'Mara et al. 2014, 2017a, b) a minimum of 6 h before the start of the first respirometry experiment. Each transmitter emits a continuous long-wave carrier signal which is interrupted by cardiac muscle potentials. We used receivers (AR8000, AOR Ltd, Tokyo, Japan) connected to digital recorders (Tascam DR-05, Los Angeles, California, USA) and recorded sound files of the time series of the cardiac muscle potentials continuously throughout experiments. Before attachment, transmitters were mounted on fabric with two wires extending through the fabric. We cut the dorsal fur at both wire insertion points, one between the shoulder blades and one in the left lumbar region. We disinfected the skin with 70% EtOH, then punctured it with a 23GA sterile needle and inserted the transmitter’s two disinfected wires ca. 5 mm under the skin (see illustration in O’Mara et al. 2017b). We glued both wires in place with surgical cement (Perma-Type Company, Plainville, Connecticut, USA) and then glued the fabric with the mounted transmitter to the back, covering the wire insertion points.
We used a custom R script to automatically identify the interruptions of the carrier signal by the muscle potentials and calculated fH in beats per minute (bpm) (O’Mara et al. 2017a; b). Automatically analyzed files were visually subsampled frequently to validate the filtering method, particularly when variation in fH was high. One observer (LK) manually counted heartbeats when automated analysis was not possible due to interference or noise.
Skin temperature monitoring
We measured Tskin with iButtons (ca. 1.6 g, DS1922L, Maxim Integrated Products, San Jose, California, USA) modified following Lovegrove (2009). The iButtons recorded Tskin every 2 min. We glued them with surgical cement near the place of insertion of the lower wire where fur was already removed. We then covered heart rate transmitters and iButtons with a second piece of fabric to prevent the animals from scratching. After completing experiments, we removed transmitters and iButtons immediately. Bats maintained body mass while wearing the equipment, suggesting no measurable negative impact of the experiments.
Respirometry and calculation of oxygen consumption
To measure metabolic rates, we used an open-flow pull through respirometry system with additional humidity control (Sable Systems International, Las Vegas, NV, USA). This setup allows for simultaneous analysis of O2, CO2 and water vapor pressure (WVP) from up to three individuals plus one empty control chamber for collecting baseline values. The setup was zeroed and spanned before each sampling season using laboratory reference gases. Bats were placed in small airtight plastic containers (volume = 800 mL) which contained a plastic grid wrapped in mesh. This allowed the bats to roost in a natural hanging position while allowing air circulation. Four mass flow systems (MFS, Sable Systems International, Las Vegas, NV, USA) with mass flow meters pulled humidity-controlled air (DG-4, Sable Systems International, Las Vegas, NV, USA) through a copper spiral for faster temperature equilibration with a constant flow rate of 150 mL/min through each of the four chambers. A subsampler (RM-8, Sable Systems International, Las Vegas, NV, USA) switched between chambers and O2, CO2 and WVP were analyzed with a field metabolic system (FMS, Sable Systems International, Las Vegas, NV, USA). We placed the respirometry chambers and the copper spiral into a climate-controlled incubator (KB53, BINDER GmbH, Tuttlingen, Germany) with a small opening on the lid to mimic the roost or bat box entrance and set the light regime in the room to the natural local circadian rhythm.
We calculated rates of oxygen consumption (\(\dot{V}{\text{O}}_{2}\)) and of CO2 production (\(\dot{V}{\text{CO}}_{2}\)) for each individual using Eqs. 11.7 and 11.8 from Lighton (2018). Before calculation of \(\dot{V}{\text{O}}_{2}\) and \(\dot{V}{\text{CO}}_{2}\), we removed the first 30 s after a channel switch and corrected for drift using a spline fit (Forsythe et al. 1977). The raw O2 and CO2 data were phase-corrected to account for the tubing connecting each of the different components. We calculated incurrent and excurrent fractional gas concentrations while correcting for water vapor dilution using Eq. 8.6 from Lighton (2018). We standardized V̇O2 based on individual body mass. We used the mean of body mass before and after the experiments in the equation to calculate \(\dot{V}{\text{O}}_{2}\) and report all \(\dot{V}{\text{O}}_{2}\) measurements in mL O2 g−1 h−1.
Data analysis and hypothesis testing
We collected data at different temporal resolutions. The \(\dot{V}{\text{O}}_{2}\) data had the lowest resolution because we switched between the respirometry chambers to measure three bats in the same experiment. This resulted in 3-min \(\dot{V}{\text{O}}_{2}\) intervals every 9 min for each bat. In each 3-min interval, we calculated the mean per minute of \(\dot{V}{\text{O}}_{2}\), fH, Tskin, and Ta. This allowed accurate conclusions about the interplay of the three different physiological parameters at changing Ta. We pooled the pre- and post-hibernation data of non-reproductive bats, as we observed no difference in the physiological states bats entered in both periods and both experiments.
We defined different physiological states by visually inspecting \(\dot{V}{\text{O}}_{2}\) over time for each individual in the 6-h and 12-h experiment. Because of the high variability in \(\dot{V}{\text{O}}_{2}\) across different Ta, we decided to use this method instead of approaches which use Tskin or \(\dot{V}{\text{O}}_{2}\) thresholds. We differentiated between torpid, resting, arousal, and torpor entry states and excluded arousal and torpor entry states for further analysis. In our experiments, at Ta < 20 °C, the torpid metabolic rate of N. noctula was 92–96% lower than metabolic rate during resting and for Ta > 20 °C 45–85% lower than metabolic rate during resting. As both ranges lie in the normal range of metabolic depression (compared to resting metabolic rate (RMR) or basal metabolic rate (BMR)) in N. noctula and other heterothermic bats (84–99% at cold temperatures (Geiser 2004), 15–93% at warm temperatures (Hosken and Withers 1997; Geiser 2004; O'Mara et al. 2017a; Reher and Dausmann 2021), we are confident that we correctly assigned physiological states.
We tested the first hypothesis in the 6-h experiment, by measuring \(\dot{V}{\text{O}}_{2}\), fH, and Tskin of reproductive and non-reproductive torpid bats when exposed to rising Ta. Some bats showed occasional arousals which were excluded from analysis as we were focused only on the torpid states. We fitted generalized linear mixed-effect models (glmer, Gamma family, log link, package lme4 (Bates et al. 2015)) to explore variation in response variables (\(\dot{V}{\text{O}}_{2}\), fH and Tskin) based on predictor variables (Ta, reproductive status) with individual (BatID) as a random factor.
We tested the second hypothesis in the 12-h experiment, by assessing the relative time reproductive and non-reproductive bats spent either in a torpid or resting state and calculating the mean energy expenditure for each bat using \(\dot{V}{\text{O}}_{2}\). We fitted generalized linear mixed-effect models (Gamma family, log link) to investigate variation in response variables (\(\dot{V}{\text{O}}_{2}\), fH, and Tskin) based on predictor variables (reproductive status and torpid or resting state) with individual as a random factor (BatID). We calculated mean ± SD for \(\dot{V}{\text{O}}_{2}\), fH and Tskin from reproductive and non-reproductive resting and torpid bats. To assess the variation in \(\dot{V}{\text{O}}_{2}\), fH and Tskin in resting bats, we calculated the coefficients of variation (CV).
Lastly, we used data from both experiments to test whether fH is a better predictor of oxygen consumption than Tskin. We fitted linear mixed-effect models (lmer) with \(\dot{V}{\text{O}}_{2}\) as the response variable and individual as a random factor (BatID) and tested if fH or Tskin would result in a better model fit. We log-transformed \(\dot{V}{\text{O}}_{2}\) to confirm equal variance and normal distribution of standardized residuals. We calculated the Akaike information criterion corrected for small sample sizes (AICc) for each model and the marginal R2 (R2m, fixed effects alone) and conditional (R2c, full model) values (Nakagawa and Schielzeth 2013) in MuMIn (Bartoń 2020). We then calculated \(\dot{V}{\text{O}}_{2}\) predictions based on fH and Tskin and compared those to measured \(\dot{V}{\text{O}}_{2}\) from the 6-h experiment, to test the model performances over a wide range of Ta.
All analyses were performed in R [Version R 4.1.3 (R Core Team 2022), RStudio Version 2022.02.1 (RStudio Team 2022)].
Results
Nyctalus noctula can maintain a reduced metabolism and heart rate at high ambient temperatures regardless of reproductive status (Hypothesis 1)
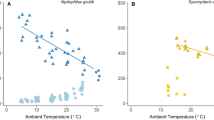
When Ta was raised in hourly increments from 0 to 32.5 °C in the 6-h experiment, we found overall low \(\dot{V}{\text{O}}_{2}\) and fH (Figs. 1a, b, S1a) in torpid reproductive and non-reproductive bats. In reproductive bats, a mean \(\dot{V}{\text{O}}_{2}\) of 0.29 ± 0.17 mL O2 g−1 h−1 (n = 9, nObservations = 193) and, in non-reproductive bats, we found a mean \(\dot{V}{\text{O}}_{2}\) of 0.26 ± 0.22 mL O2 g−1 h−1 (n = 19, nObservations = 491). The predicted relationship between \(\dot{V}{\text{O}}_{2}\) and Ta for reproductive bats was \(\dot{V}{\text{O}}_{2}\) = exp(− 1.86 + 0.03 × Ta) and for non-reproductive bats \(\dot{V}{\text{O}}_{2}\) = exp(− 2.05 + 0.03 × Ta). In reproductive bats, a mean fH of 64 ± 34 bpm (n = 9, nObservations = 164) and, in non-reproductive bats, we found a mean fH of 55 ± 36 bpm (n = 19, nObservations = 484). The predicted relationship between fH and Ta for reproductive bats was fH = exp(3.48 + 0.04 × Ta) and for non-reproductive bats fH = exp(3.25 + 0.04 × Ta). Bats thermoconformed to Ta (Fig. 1c) and we recorded a wide range of Tskin (reproductive: 2.9–30.9 °C, n = 9, nObservations = 182; non-reproductive: 1.1–32.2 °C, n = 12, nObservations = 256). The predicted relationship between Tskin and Ta for reproductive bats was Tskin = exp(1.82 + 0.06 × Ta) and for non-reproductive bats Tskin = exp(1.80 + 0.06 × Ta). There were occasional arousals (Fig. S1b) which were excluded from analysis. Overall, Ta explained the variation in response variables (\(\dot{V}{\text{O}}_{2}\), fH and Tskin) while reproductive status had no significant effect (Table S1).
Irrespective of reproductive status (green = non-reproductive, yellow = reproductive), N. noctula remained torpid when exposed to rising Ta. a \(\dot{V}{\text{O}}_{2}\) increased slightly but remained low across all Ta (non-reproductive: n = 19, nObservations = 491; reproductive: n = 9, nObservations = 193). Grey dashed line indicates the BMR of N. noctula = 1.47 mL O2 g−1 h−1 (reported in Geiser 2004). b fH increased slightly but remained low across a wide range of Ta (non-reproductive: n = 19, nObservations = 484; reproductive: n = 9, nObservations = 164). c Tskin increased with rising Ta when torpid bats thermoconformed (non-reproductive: n = 12, nObservations = 256; reproductive: n = 9, nObservations = 182). Shaded green and yellow areas indicate the 95% CI
Under natural temperature conditions, reproductive bats use torpor less frequently than non-reproductive bats (Hypothesis 2)
We exposed bats to seasonal Ta in the 12-h experiment to assess the physiological responses in non-reproductive and reproductive bats. After a short arousal at the beginning of the experiment, all non-reproductive bats (n = 19) had low Tskin, fH, and \(\dot{V}{\text{O}}_{2}\) and thermoconformed to Ta throughout the experiment (“Only torpid”) (Fig. 2a). In reproductive bats (n = 12), we found three different torpor use strategies: Four individuals rested throughout the 12-h experiment (“Only resting”, Fig. 2b) and had the highest mean \(\dot{V}{\text{O}}_{2}\) ± SD, three individuals exclusively used torpor throughout the experiment (“Only torpid”) and had 93% energy savings compared to the “only resting” bats and five individuals used a combination of resting and torpor (“Combination”, Fig. S2) which resulted in lower mean \(\dot{V}{\text{O}}_{2}\) ± SD and 33% energy savings (Table 1).
Different torpor use strategies in non-reproductive and reproductive female N. noctula in the 12-h experiment. a Representative figure of a non-reproductive bat using the “only torpid” strategy. Upper panel: \(\dot{V}{\text{O}}_{2}\) (black dashed line) and fH (pink solid line) were lowered after a short arousal at the beginning of the experiment. Lower panel: the bat thermoconformed Tskin (light-blue solid line) to Ta (dark-blue dashed line). b Representative figure of a reproductive bat using the “only resting” strategy. Upper panel: \(\dot{V}{\text{O}}_{2}\) (black dashed line) and fH (pink solid line) were very variable. Lower panel: the bat thermoregulated and Tskin (light-blue solid line) was constantly higher than Ta (dark-blue dashed line)
Reproductive status and whether the bats were in a torpid or resting state explained variation in \(\dot{V}{\text{O}}_{2}\), fH and Tskin with significant differences between resting reproductive, torpid reproductive and torpid non-reproductive individuals (Table S2). Mean ± SD of \(\dot{V}{\text{O}}_{2}\), fH and Tskin was highest in resting reproductive individuals. In torpid bats, mean ± SD of \(\dot{V}{\text{O}}_{2}\) and fH were higher in reproductive individuals, compared to non-reproductive individuals (Table 2). The differences in Tskin in torpid bats were explained by the different Ta the bats were exposed to in each season. Resting individuals had the highest and most variable \(\dot{V}{\text{O}}_{2}\) with a coefficient of variation (CV) of 22.66 and fH with a CV of 16.23 compared to Tskin with a CV of 7.93.
Heart rate as a predictor of oxygen consumption
We fitted linear mixed-effect models based on data from both experiments to verify that fH is a better predictor of \(\dot{V}{\text{O}}_{2}\) than Tskin. The model that included fH (fH model) had a high R2c (0.83) and lower AICc and explained the variation in \(\dot{V}{\text{O}}_{2}\) well. The model that included Tskin (Tskin model) had a lower predictive ability for \(\dot{V}{\text{O}}_{2}\) (Table S3). We then predicted values from the Tskin model (traditionally used method) and the fH model and compared them to measured \(\dot{V}{\text{O}}_{2}\) values from bats in the 6-h experiment. When Ta > 20 °C, the Tskin model overpredicted \(\dot{V}{\text{O}}_{2}\) while the fH model better predicted \(\dot{V}{\text{O}}_{2}\) (Fig. 3).
Predictions for \(\dot{V}{\text{O}}_{2}\) from the Tskin model and the fH model and the measured \(\dot{V}{\text{O}}_{2}\) for one representative bat in the 6-h experiment with rising Ta. a Predictions for \(\dot{V}{\text{O}}_{2}\) from the fH model (pink dashed line) were similar to the measured \(\dot{V}{\text{O}}_{2}\) (black solid line). Predictions for \(\dot{V}{\text{O}}_{2}\) from the Tskin model (light-blue dotted line) overpredicted \(\dot{V}{\text{O}}_{2}\) when Ta was raised above 20 °C. b Ta (dark-blue solid line) was raised every hour and was > 20 °C after 09:00
Discussion
Consistent with our hypotheses, we found frequent use of torpor at high Ta with low \(\dot{V}{\text{O}}_{2}\) and fH but high Tskin, in our temperate-zone bats. We demonstrated the wide-ranging energetic consequences of the variability in torpor use on reproductive animals and confirmed that fH is a better predictor for \(\dot{V}{\text{O}}_{2}\) than Tskin.
At high Ta, female N. noctula reduced \(\dot{V}{\text{O}}_{2}\) by up to 85% compared to BMR (reported in Geiser 2004) (Fig. 1a) while fH was reduced to 1/8 of resting fH (Fig. 1b). Although Tskin remained high (Fig. 1c), this physiological state would be defined as torpor (Geiser 2021). A similar torpid state at high Ta is known from tropical bat species (M. molossus: 60% decrease in metabolic rate and 90% in fH (O'Mara et al. 2017a) or M. commersoni [80% decrease in metabolic rate, fH not measured (Reher and Dausmann 2021)], but has never been described in a temperate-zone bat. Heterothermy most likely evolved in the tropics and is considered to be an evolutionary stage between the ancestral ectothermy and the endothermy found in most mammal species (Grigg et al. 2004; Lovegrove 2017). It is, therefore, possible that temperate-zone bat species also preserved the physiological ability to express torpor at higher Ta, despite usually experiencing cooler Ta than their tropical counterparts.
The physiological mechanisms which allow N. noctula to maintain a reduced \(\dot{V}{\text{O}}_{2}\) and fH at high Tskin are unknown. In our experiment, bats entered torpor at cold Ta, but Tskin was decoupled from fH and \(\dot{V}{\text{O}}_{2}\) with rising Ta. We observed a slight increase in fH and \(\dot{V}{\text{O}}_{2}\) with rising Ta; however, \(\dot{V}{\text{O}}_{2}\) at 32.5 °C was still far below the BMR (Figs. 1a, S1). This suggests that the reduced metabolism observed during torpor was not just a consequence of passive thermal effects (Guppy and Withers 1999), where one would expect that increases in Ta and Tskin translate directly to increased \(\dot{V}{\text{O}}_{2}\). Instead, the reduced metabolism was likely a consequence of an active metabolic depression (Heldmaier et al. 2004; Geiser 2021). It remains unknown if tropical and temperate-zone bat species use the same underlying physiological processes for this. Additionally, while cardiac function during torpor at low body temperatures has been investigated (Milsom et al. 2001; Currie et al. 2018), the dynamics of blood pressure, blood viscosity, stroke volume and heart muscle function when the heart performs at different paces and extreme differences in body temperatures have not been evaluated.
We exposed captive bats to rising Ta with an increase of 32.5 °C within 6 h; a scenario which is unlikely to occur naturally. Nevertheless, the fact that reproductive and non-reproductive individuals had the physiological ability to decouple fH and \(\dot{V}{\text{O}}_{2}\) from Tskin suggests that this strategy is also available to bats in the wild. The maximum heat tolerance in N. noctula is unknown, but during summer N. noctula seem to prefer natural tree cavities over artificial roosting boxes in Konstanz (personal observation). They possibly avoid high Ta with this behavior, as tree-cavity roosting temperate-zone bat species generally have lower heat tolerance (Noakes et al. 2021) and at the population level, high summer daytime temperatures are associated with higher mortality (Mundinger et al. 2021). However, in a population in Białowieża, Poland, pregnant females prefer warmer maternity roosts, potentially to decrease energy expenditure when remaining normothermic (Ruczyński 2006; Ruczyński and Bartoń 2020). The factors which determine when a bat uses torpor at high body temperatures are unknown, but we suggest that using torpor at higher Ta is energetically less costly and reduces water loss compared to maintaining a high metabolism. Further study with a combination of monitoring roost temperatures and fH of free-ranging bats could help quantify how often bats are exposed to higher Ta and to what extent they use torpor as an energy-saving strategy.
When we exposed bats to simulated Ta in the 12-h experiment, all non-reproductive bats used torpor throughout (Fig. 2a), whereas reproductive bats used different torpor strategies (Table 1, Figs. 2b, S2). Notably, following outside temperatures, we did not expose the bats to Ta higher than 18.6 °C in the 12-h experiment and they did not use torpor at high Ta. The fact that reproductive bats use less torpor than non-reproductive ones is well known and most likely due to negative impacts on foetal development or milk production (Hamilton and Barclay 1994; Grinevitch et al. 1995; Dietz and Kalko 2006; Rambaldini and Brigham 2008; Dietz and Hörig 2011; Baloun and Guglielmo 2019). It is more difficult to ascertain why there is individual variation in torpor use in reproductive individuals (Rambaldini and Brigham 2008; Besler and Broders 2019). In other heterothermic species, different torpor use strategies within one reproductive stage are the result of various factors such as Ta (Geiser and Broome 1993; Dausmann et al. 2020), roost type (Rintoul and Brigham 2014), foraging success or food availability (Canale et al. 2011; Vuarin and Henry 2014; Komar et al. 2020), genetic variation in torpor related traits and body condition (Lane et al. 2011; Vuarin et al. 2013), state of pregnancy (Besler and Broders 2019), age (Bieber et al. 2018), and differences in personality or stress responses under laboratory conditions (Ruf and Geiser 2015). In our experiment, all bats from the same season were fed ad libitum and exposed to identical Ta and light and yet reproductive females differed in torpor use. We did not find any common characteristics, such as body condition or age among the three reproductive females which always used torpor, which would explain why their strategy differed from the other reproductive bats. We need further research to fully understand if and how body condition, age, stress or genetic variation affects torpor use strategies in heterothermic animals.
The different torpor use strategies we observed resulted in large differences in energy expenditure. Reproductive females using torpor had slightly higher fH and \(\dot{V}{\text{O}}_{2}\) than torpid non-reproductive individuals (Table 2). This could be a consequence of differences in metabolic rates between reproductive and non-reproductive female bats (McLean and Speakman 2000). However, when kept under identical thermal conditions, torpid metabolic rate did not differ across reproductive stages in another bat species (Turbill and Geiser 2006), and it is likely that the differences in metabolic rate are a consequence of the higher Ta (matching seasonal ambient conditions) reproductive bats were exposed to in the 12-h experiment. “Only torpid” reproductive individuals saved 93% of their daily energy expenditure compared to “only resting” individuals and the bats which used the “combination” strategy saved 33% (Table 1). This shows that when reproductive individuals use torpor even for part of the day, they can save large amounts of energy while potentially keeping the detrimental effects on reproduction low. One possibility to explain the variation in our results is that females may flexibly allocate energy to foetal growth based on individual energetic levels. Indeed, Tskin monitoring in free-ranging individuals indicates that female bats flexibly choose a torpor use strategy and opportunistically use torpor to decrease energy expenditure in different reproductive states depending on environmental conditions and possibly feeding success (Lausen and Barclay 2003; Dzal and Brigham 2013).
Even though our results from captive bats seem to fit observations of torpor use in free-ranging bats, we cannot exclude the possibility that captivity affected the bats' torpor use (Geiser et al. 2000). We, therefore, want to emphasize the importance to study free-ranging individuals to reveal the full potential of the various strategies bats use to optimize energy expenditure. fH has been used successfully to monitor torpor of hibernators such as ground squirrels (Milsom et al. 1999; MacCannell et al. 2018) or brown bears (Evans et al. 2016) in the wild, and in the past years, equipment has become small and light enough to monitor fH in free-ranging bats (O'Mara et al. 2017a, b). We show that in both torpid and normothermic bats, changes in fH are almost immediately reflected in \(\dot{V}{\text{O}}_{2}\) while Tskin follows more slowly and with a delay, if at all (Figs. 2, S1, S2). In both experiments, across a wide range of Tskin, \(\dot{V}{\text{O}}_{2}\) was strongly correlated with fH (Figs. 1, 2) and the fH model predicted \(\dot{V}{\text{O}}_{2}\) better than the Tskin model (Table S3). Our data show that models based on measurements of Tskin allow accurate predictions for N. noctula when Ta are below 20 °C, but the error increases rapidly at higher Ta (Fig. 3).
Flexibility in torpor use might be key to helping heterothermic species overcome energetic challenges during reproduction and minimize the threat of extinction in an increasingly warmer world (Geiser and Turbill 2009; Canale et al. 2011; Levesque et al. 2016). Using fH monitoring, future research can accurately quantify how the use of torpor at high body temperatures and flexible torpor use translates in daily energy expenditures in free-ranging heterothermic species. This will bring us one step closer to reveal the full extent of behavioral and physiological adaptations animals use to survive.
Data availability
Data files associated with this study are deposited in the Open Research Data Repository of the Max Planck Society “Edmond” (https://doi.org/10.17617/3.T5LUIM).
References
Adams RA (2010) Bat reproduction declines when conditions mimic climate change projections for western North America. Ecology 91:2437–2445
Audet D, Fenton MB (1988) Heterothermy and the use of torpor by the bat Eptesicus fuscus (Chiroptera: Vespertilionidae): a field study. Physiol Zool 61:197–204
Baloun DE, Guglielmo CG (2019) Energetics of migratory bats during stopover: a test of the torpor-assisted migration hypothesis. J Exp Biol 222(Pt 6):jeb196691. https://doi.org/10.1242/jeb.196691
Barclay MRR, Kalcounis MC, Crampton LH, Stefan C, Vonhof MJ, Wilkinson L, Brigham RM (1996) Can external radiotransmitters be used to assess body temperature and torpor in bats? J Mammal 77:1102–1106
Bartoń,K (2020) MuMIn: multi-Model Inference. R package version 1.43.17
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:48
Besler NK, Broders HG (2019) Combinations of reproductive, individual, and weather effects best explain torpor patterns among female little brown bats (Myotis lucifugus). Ecol Evol 9:5158–5171
Bieber C, Turbill C, Ruf T (2018) Effects of aging on timing of hibernation and reproduction. Sci Rep 8:13881
Bondarenco A, Körtner G, Geiser F (2014) Hot bats: extreme thermal tolerance in a desert heat wave. Naturwissenschaften 101:679–685
Braun M, Dieterlen F (2003) Die Säugetiere Baden-Württembergs. Band 1: Allgemeiner Teil, Fledermäuse (Chiroptera). Verlag Eugen Ulmer, Stuttgart
Canale CI, Perret M, Théry M, Henry P-Y (2011) Physiological flexibility and acclimation to food shortage in a heterothermic primate. J Exp Biol 214:551–560
Currie SE, Stawski C, Geiser F (2018) Cold-hearted bats: uncoupling of heart rate and metabolism during torpor at sub-zero temperatures. J Exp Biol 221:jeb170894
Dausmann KH, Levesque DL, Wein J, Nowack J (2020) Ambient temperature cycles affect daily torpor and hibernation patterns in Malagasy tenrecs. Front Physiol 11:522. https://doi.org/10.3389/fphys.2020.00522
Dechmann DK, Ehret S, Gaub A, Kranstauber B, Wikelski M (2011) Low metabolism in a tropical bat from lowland Panama measured using heart rate telemetry: an unexpected life in the slow lane. J Exp Biol 214:3605–3612
Dechmann DK, Wikelski M, Varga K, Yohannes E, Fiedler W, Safi K, Burkhard W-D, O’Mara MT (2014) Tracking post-hibernation behavior and early migration does not reveal the expected sex-differences in a “female-migrating” bat. PLoS ONE 9:e114810
Dechmann DKN, Wikelski M, Ellis-Soto D, Safi K, O’Mara MT (2017) Determinants of spring migration departure decision in a bat. Biol Lett 13:20170395
Dietz M, Hörig A (2011) Thermoregulation of tree-dwelling temperate bats—a behavioural adaptation to force live history strategy. Folia Zool 60(5–16):12
Dietz M, Kalko EK (2006) Seasonal changes in daily torpor patterns of free-ranging female and male Daubenton’s bats (Myotis daubentonii). J Comp Physiol B 176:223–231
Dzal YA, Brigham RM (2013) The tradeoff between torpor use and reproduction in little brown bats (Myotis lucifugus). J Comp Physiol B 183:279–288
Evans AL, Singh NJ, Friebe A, Arnemo JM, Laske TG, Fröbert O, Swenson JE, Blanc S (2016) Drivers of hibernation in the brown bear. Front Zool 13:7
Forsythe GE, Malcolm MA, Moler CB (1977) Computer methods for mathematical computations. Prentice-Hall series in automatic computation. ISBN: 978-0131653320
Geiser F (2004) Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66:239–274
Geiser F (2021) Ecological physiology of daily torpor and hibernation, fascinating Life Sciences. Springer Nature AG
Geiser F, Broome LS (1993) The effect of temperature on the pattern of torpor in a marsupial hibernator. J Comp Physiol B 163:133–137
Geiser F, Turbill C (2009) Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften 96:1235–1240
Geiser F, Holloway JC, Körtner G, Maddocks TA, Turbill C, Brigham RM (2000) Do patterns of torpor differ between free-ranging and captive mammals and birds? In: Heldmaier G, Klingenspor M (eds) Life in the cold. Springer, Berlin, Heidelberg, pp 95–102. https://doi.org/10.1007/978-3-662-04162-8_10
Grigg GC, Beard LA, Augee ML (2004) The evolution of endothermy and its diversity in mammals and birds. Physiol Biochem Zool 77:982–997
Grinevitch L, Holroyd SL, Barclay RMR (1995) Sex differences in the use of daily torpor and foraging time by big brown bats (Eptesicus fuscus) during the reproductive season. J Zool 235:301–309
Guppy M, Withers P (1999) Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol Rev 74:1–40
Hamilton I, Barclay R (1994) Patterns of daily torpor and day-roost selection by male and female big brown bats (Eptesicus fuscus). Can J Zool 72:744–749
Heldmaier G, Ortmann S, Elvert R (2004) Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol 141:317–329
Hosken DJ, Withers PC (1997) Temperature regulation and metabolism of an Australian bat, Chalinolobus gouldii (Chiroptera: Vespertilionidae) when euthermic and torpid. J Comp Physiol B 167:71–80
Jonasson KA, Willis CK (2012) Hibernation energetics of free-ranging little brown bats. J Exp Biol 215:2141–2149
Komar E, Dechmann DKN, Fasel NJ, Zegarek M, Ruczyński I (2020) Food restriction delays seasonal sexual maturation but does not increase torpor use in male bats. J Exp Biol 223:jeb214825
Kunz TH (1982) Roosting ecology of bats. In: Kunz TH (ed) Ecology of bats. Springer, Boston, pp 1–55. https://doi.org/10.1007/978-1-4613-3421-7_1
Kunz TH, Wrazen JA, Burnett CD (1998) Changes in body mass and fat reserves in pre-hibernating little brown bats (Myotis lucifugus). Ecoscience 5:8–17
Lane J, Kruuk L, Charmantier A, Murie J, Coltman D, Buoro M, Raveh S, Dobson F (2011) A quantitative genetic analysis of hibernation emergence date in a wild population of Columbian ground squirrels. J Evol Biol 24:1949–1959
Lausen CL, Barclay RMR (2003) Thermoregulation and roost selection by reproductive female big brown bats (Eptesicus fuscus) roosting in rock crevices. J Zool 260:235–244
Lehnert LS, Kramer-Schadt S, Teige T, Hoffmeister U, Popa-Lisseanu A, Bontadina F, Ciechanowski M, Dechmann DK, Kravchenko K, Presetnik P (2018) Variability and repeatability of noctule bat migration in Central Europe: evidence for partial and differential migration. Proc R Soc B 285:20182174
Levesque DL, Nowack J, Stawski C (2016) Modelling mammalian energetics: the heterothermy problem. Clim Change Responses 3:7
Lighton JR (2018) Measuring metabolic rates: a manual for scientists. Oxford University Press, Oxford
Lovegrove BG (2009) Modification and miniaturization of Thermochron iButtons for surgical implantation into small animals. J Comp Physiol B 179:451–458
Lovegrove BG (2017) A phenology of the evolution of endothermy in birds and mammals. Biol Rev 92:1213–1240
MacCannell AD, Jackson EC, Mathers KE, Staples JF (2018) An improved method for detecting torpor entrance and arousal in a mammalian hibernator using heart rate data. J Exp Biol 221:jeb174508
Maloney SK, Bronner GN, Buffenstein R (1999) Thermoregulation in the Angolan free-tailed bat Mops condylurus: a small mammal that uses hot roosts. Physiol Biochem Zool 72:385–396
McLean JA, Speakman JR (2000) Effects of body mass and reproduction on the basal metabolic rate of brown long-eared bats (Plecotus auritus). Physiol Biochem Zool 73:112–121
Milsom WK, Zimmer MB, Harris MB (1999) Regulation of cardiac rhythm in hibernating mammals. Comp Biochem Physiol A Mol Integr Physiol 124:383–391
Milsom WK, Zimmer MB, Harris MB (2001) Vagal control of cardiorespiratory function in hibernation. Exp Physiol 86:791–796
Mundinger C, Scheuerlein A, Kerth G (2021) Long-term study shows that increasing body size in response to warmer summers is associated with a higher mortality risk in a long-lived bat species. Proc R Soc B Biol Sci 288:20210508
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Noakes MJ, McKechnie AE, Brigham RM (2021) Interspecific variation in heat tolerance and evaporative cooling capacity among sympatric temperate-latitude bats. Can J Zool 99:480–488
O’Mara MT, Wikelski M, Dechmann DKN (2014) 50 years of bat tracking: device attachment and future directions. Methods Ecol Evol 5:311–319
O’Mara MT, Rikker S, Wikelski M, Ter Maat A, Pollock HS, Dechmann DKN (2017a) Heart rate reveals torpor at high body temperatures in lowland tropical free-tailed bats. R Soc Open Sci 4:171359
O’Mara MT, Wikelski M, Voigt CC, Ter Maat A, Pollock HS, Burness G, Desantis LM, Dechmann DKN (2017b) Cyclic bouts of extreme bradycardia counteract the high metabolism of frugivorous bats. Elife 6:e26686
R Core Team (2022) R: A LANGUAGE and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rambaldini DA, Brigham RM (2008) Torpor use by free-ranging Pallid Bats (Antrozous pallidus) at the Northern extent of their range. J Mammal 89:933–941
Reher S, Dausmann KH (2021) Tropical bats counter heat by combining torpor with adaptive hyperthermia. Proc R Soc B Biol Sci 288:20202059
Rintoul JL, Brigham RM (2014) The influence of reproductive condition and concurrent environmental factors on torpor and foraging patterns in female big brown bats (Eptesicus fuscus). J Comp Physiol B 184:777–787
RStudio Team (2022) RStudio: integrated development environment for R. RStudio, PBC, Boston
Ruczyński I (2006) Influence of temperature on maternity roost selection by noctule bats (Nyctalus noctula) and Leisler’s bats (N. leisleri) in Biaowieza Primeval Forest, Poland. Can J Zool 84:900–907
Ruczyński I, Bartoń KA (2020) Seasonal changes and the influence of tree species and ambient temperature on the fission-fusion dynamics of tree-roosting bats. Behav Ecol Sociobiol 74:63
Ruczyński I, Kalko EK, Siemers BM (2007) The sensory basis of roost finding in a forest bat, Nyctalus noctula. J Exp Biol 210:3607–3615
Ruczyński I, Hałat Z, Zegarek M, Borowik T, Dechmann DK (2020) Camera transects as a method to monitor high temporal and spatial ephemerality of flying nocturnal insects. Methods Ecol Evol 11:294–302
Ruf T, Geiser F (2015) Daily torpor and hibernation in birds and mammals. Biol Rev Camb Philos Soc 90:891–926
Schmidt-Nielsen K (1970) Energy metabolism, body size, and problems of scaling. Fed Proc 29(4):1524–1532
Stawski C, Willis CKR, Geiser F (2014) The importance of temporal heterothermy in bats. J Zool 292:86–100
Turbill C, Geiser F (2006) Thermal physiology of pregnant and lactating female and male long-eared bats, Nyctophilus geoffroyi and N. gouldi. J Comp Physiol B 176:165–172
Vuarin P, Henry P-Y (2014) Field evidence for a proximate role of food shortage in the regulation of hibernation and daily torpor: a review. J Comp Physiol B 184:683–697
Vuarin P, Dammhahn M, Henry PY (2013) Individual flexibility in energy saving: body size and condition constrain torpor use. Funct Ecol 27:793–799
Acknowledgements
We would like thank Klaus Heck, Wolfgang Fiedler and Alexandra Sproll for sharing locations and information about N. noctula roosts. We thank the staff of the Insel Mainau and the Fledermauskoordinationsstelle Thurgau for their friendly cooperation and access to bat boxes. Marion Muturi helped with fieldwork and Stephen Albert Tyndel gave valuable feedback on previous drafts. We thank Teague O’Mara for sharing his R script for the automated analysis of heart rates and Bernd Vorneweg for valuable input and help improving the heart rate transmitters. We thank the “Studienstiftung des Deutschen Volkes” for funding the PhD project of LK.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Max Planck Society, the University of Konstanz and the “Studienstiftung des Deutschen Volkes”.
Author information
Authors and Affiliations
Contributions
LK, PJS and DKND conceived the ideas and designed the methodology; LK, IR and EK conducted the field work and collected the data; LK and JRS analyzed the data; LK led the writing of the manuscript. All the authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All experimental procedures conducted in Poland were authorized by the Regional Director for Environmental Protection in Białystok (authorization number WPN.6401.131.2019.MC) and by the Local Ethical Commissions in Białystok and Olsztyn (authorization number 26/2019). All experimental procedures in Germany were authorized by the Local Ethical Commission in Freiburg (authorization number 35–9185.81/G-18/99).
Additional information
Communicated by K.H. Dausmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keicher, L., Shipley, J.R., Komar, E. et al. Flexible energy-saving strategies in female temperate-zone bats. J Comp Physiol B 192, 805–814 (2022). https://doi.org/10.1007/s00360-022-01452-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-022-01452-7