Abstract
Hematologic abnormalities are common manifestations of SLE, although neutropenia is observed less frequently and is not included in the classification criteria. Nonetheless, neutropenia is a risk factor for infections, especially those caused by bacteria or fungi. We aimed to evaluate the impact of neutropenia in SLE through a systematic investigation of all infections in a large cohort of well-characterized patients, focusing on neutropenia, lymphopenia, and hypocomplementemia. Longitudinal clinical and laboratory parameters obtained at visits to the Rheumatology Unit, Linköping University Hospital, and linked data on all forms of healthcare utilization for all the subjects included in our regional SLE register during 2008–2022 were assessed. Data regarding confirmed infections were retrieved from the medical records. Overall, 333 patients were included and monitored during 3,088 visits to a rheumatologist during the study period. In total, 918 infections were identified, and 94 occasions of neutropenia (ANC < 1.5 × 109/L) were detected in 40 subjects (12%). Thirty neutropenic episodes in 15 patients occurred in association with infections, of which 13 (43%) required in-hospital care, 4 (13%) needed intensive care, and 1 (3%) resulted in death. Bayesian analysis showed that patients with ≥ 1 occasion of neutropenia were more likely to experience one or more infections (OR = 2.05; probability of association [POA] = 96%). Both invasiveness (OR = 7.08; POA = 98%) and severity (OR = 2.85; POA = 96%) of the infections were significantly associated with the present neutropenia. Infections are common among Swedish SLE patients, 12% of whom show neutropenia over time. Importantly, neutropenia is linked to both the invasiveness and severity of infections. Awareness of the risks of severe infections in neutropenic patients is crucial to tailor therapies to prevent severe illness and death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical spectrum of systemic lupus erythematosus (SLE) is highly heterogeneous, ranging from mild disease, which can be limited to skin and joint involvement, to life-threatening conditions that may involve renal impairment, severe cytopenia, central nervous system disease, and thromboembolic events [1]. Thus, SLE continues to represent a major challenge for both patients and physicians. Inappropriate or incomplete management of SLE disease activity and/or the side effects of administered therapies, particularly corticosteroids, may lead to comorbidities, irreversible organ damage, decreased health-related quality of life, and increased mortality [2,3,4].
Infections remain one of the leading causes of mortality and morbidity for patients with SLE [5]. The increased susceptibility to infections is related to the disease itself, as well as to the administered medications. SLE is associated with an increased risk of infections due to altered host immune status, i.e., hypocomplementemia, as well as due to abnormal neutrophil and macrophage responses to pathogens. In addition, immunosuppressive drugs, such as corticosteroids, cytotoxic agents and biologics, may affect the lymphocyte count as well as B and T cell functions and thereby impair the immune response to viral and bacterial pathogens, further contributing to the risk of infections [5,6,7,8].
Data from the USA, the UK, and Singapore indicate that approximately 50% of patients with SLE experience at least one severe infection during their disease course, and that 11%–23% of all hospitalizations among individuals with SLE are due to infections [9,10,11]. A recent study conducted by Simard et al. based on Swedish registry data found that individuals with incident SLE were two to four times more likely to be hospitalized for infections and experienced more recurrent infections than the general population. Among immunosuppressive agents, azathioprine was associated with the highest rate of infections [12]. Another European registry study has shown that bacterial infections account for 52% of all infections in patients with SLE, followed by viruses (12%) and fungi (2%) [13]. Regarding sites of infections, the respiratory tract (35%), urinary tract (15%), and soft tissues (13%) have been reported among individuals with SLE [13, 14]. However, although many bacterial infections are evidently more prevalent in patients with SLE than in the general population, the causal pathogens do not seem to differ much and include mainly Staphylococcus aureus, Streptococcus pneumonia, and Escherichia coli [5]. However, the rates of opportunistic infections may be underestimated in patients with SLE due to their similarities with disease flares [15, 16].
Hematologic abnormalities occur frequently in patients with SLE and are included in the classification criteria [17, 18]. Lymphopenia is particularly common and is associated with an increased risk of damage accrual [19]. However, cytopenias can have several etiologies, including disease activity, bone marrow failure, drug toxicity, peripheral cell destruction, tumor infiltration, and sepsis [20, 21]. Neutropenia is found less often than other cytopenias in patients with SLE [22]. The putative mechanism behind autoimmune neutropenia involves increased peripheral cell destruction by circulating anti-neutrophil antibodies [23], increased margination, changes in the marginal zone splenic cell pools [24], and decreased granulopoiesis, but other mechanisms may also be of relevance, e.g., drugs and infections [25, 26]. Most episodes of neutropenia in SLE are mild, with an absolute neutrophil count (ANC; < 1.5‒ ≥ 1.0 × 109/L), although approximately 5% of patients may experience moderate (ANC < 1.0‒ ≥ 0.5 × 109/L) to severe (ANC < 0.5‒ ≥ 0.2 × 109/L) neutropenia [25]. Low serum levels of soluble Fc-γ receptor and high levels of granulocyte colony-stimulating factor have been proposed as risk factors for the development of infections in neutropenic patients with SLE [27, 28]. Furthermore, neutrophil function has also been demonstrated to be of high relevance in SLE [29].
The aims of this study were to; (i) identify systematically and characterize, over a period of 14 years, all the infections that occurred in a large cohort of Swedish patients with confirmed SLE; and (ii) explore any differences in infections that occurred in conjunction with or in the absence of neutropenia in these patients with SLE.
Patients and methods
Subjects
The patients in this retrospective observational study originated from the regional research program Clinical Lupus Register in Northeastern Gothia (Swedish acronym: KLURING) and fulfilled the 1982 American College of Rheumatology (ACR) and/or the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria [17, 18]. This single-center cohort has recently been described in detail and includes all adult individuals (≥ 18 years of age) with SLE residing in Östergötland County; patients were continuously enrolled as prevalent or incident cases and longitudinally followed since 2008 at the Rheumatology Unit, Linköping University Hospital [30]. The current study encompassed all the included subjects followed prospectively until death, April 30th 2022 or emigration from the study region. Data on all healthcare consumption in the study region were available to us. At all visits to the rheumatologist, ongoing medications were registered, and disease activity was assessed using the clinical SLE disease activity index 2000 (SLEDAI-2 K) with exclusion of items for anti-dsDNA binding and hypocomplementemia. Blood samples for blood cell counts and assays for creatine kinase and complement protein (C)3 and C4, as well as urinalysis were monitored at each visit to the rheumatologist [30].
Identification of neutropenia
During the healthcare visits, episodes of neutropenia, defined as ANC < 1.5 × 109/L, were identified. This cutoff has been applied in previous studies of adults [31, 32]. Furthermore, with regard to the definitions derived from previous studies, neutropenia was here categorized as: mild, for ANC < 1.5‒ ≥ 1.0 × 109/L; moderate, for ANC < 1.0‒ ≥ 0.5 × 109/L; severe, for ANC < 0.5‒ ≥ 0.2 × 109/L; and agranulocytosis, for ANC < 0.2 × 109/L. In addition, the episodes of neutropenia were classified as either ‘occasional’ or ‘recurrent’. When one or more tests displayed neutropenia within a single 4-week time span, the neutropenia was considered to be occasional. If the neutropenia reoccurred over several 4-week time spans (at least two episodes), the neutropenia was considered to be recurrent.
Identification and validation of infections
We conducted a systematic case record review of each included patient from the date of inclusion in KLURING until death, April 30th 2022, or emigration from the study region. The review was initially performed by M.S., consultant rheumatologist, and then further reviewed by J.S., senior consultant in infectious diseases. The collected data concerned the public healthcare utilization in the study region of all the included patients, reflecting infection-related complaints or symptoms (from either physical in-person or digital contacts with healthcare professionals). We included only those infections where the diagnosis was made by a physician based on typical symptoms, physical examinations, and laboratory, culture, or radiologic findings, and the diagnosis was required to have been documented, given a relevant ICD-10 code, and treated as an infectious disease. Data on the use of antibiotics as well as antiviral and antifungal drugs were available, including both prescriptions and agents provided in-hospital care.
Characterization of infections
The identified infections were assigned to the following categories: (i) bacterial, viral, fungal, or mixed infections; (ii) affected organ system or localizations: bone, skin, and soft tissues, and respiratory, urogenital, gastrointestinal, neurologic, or multiple organ systems, in addition to infections with unspecified localizations; (iii) invasive and non-invasive infections (when the microorganism was found in the blood or in the cerebrospinal fluid); and (iv) the severity of the infections, depending on how they were treated and managed. Infection severity was rated on four levels: (1) infections treated in the outpatient care setting; (2) infections requiring in-hospital care; (3) infections necessitating intensive care; (4) and infections causing death.
The identified infections were divided into three subgroups: (a) infections that occurred in conjunction with confirmed neutropenia; (b) infections that occurred in the absence of neutropenia; and (c) infections in which neutropenia had not been verified with a white blood cell (WBC) differentiation test 2 months prior to or after the infectious episode.
For all neutropenic episodes, regardless of concomitant infection, information regarding hypocomplementemia and lymphopenia was available and neutrophil-to-lymphocyte ratio (NLR) could be provided. Hypocomplementemia was defined as C3 ≤ 0.69 g/mL and/or C4 ≤ 0.12 g/mL, according to the local laboratory at Linköping University Hospital, and lymphopenia was defined as a lymphocyte count < 1.1 × 109/L. To increase the statistical power, fulfillment of the SLICC classification criteria for ‘lymphopenia’ and ‘low complement’ was used instead of concomitant hypocomplementemia and lymphocyte count in specified analyses [17].
Statistics
To characterize the infections identified in the case review, we report descriptive statistics for infection type, location, invasiveness, and severity. Logistic regression analysis was used to estimate the odds of infection based on ever having experienced neutropenia, and for estimating the odds of infection when neutropenic episodes occurred in patients with ongoing or a history of hypocomplementemia and lymphopenia. For the models of co-occurrence, we added an adaptive intercept to the models, to account for the clustering of infections within patients. We also analytically estimated the associations between infection invasiveness and severity with co-occurring neutropenia, adjusted for whether patients had ever experienced hypocomplementemia and lymphopenia during the study period. In these models, we also added an interaction term between hypocomplementemia and lymphopenia (according to the SLICC criteria) [17]. We used ordinal regression for severity and logistic regression for invasiveness (infections for which no culture had been assessed were removed), with adaptive intercepts introduced to account for the clustering of infections within patients.
All the regression models were estimated using Bayesian inference [33]. We used Student t-distributions as priors for the intercepts and coefficients (degrees of freedom = 3, center = 0, scale = 2.5). We present the medians of the posterior distributions as point estimates, along with the 95% compatibility intervals (CI), defined by the 2.5% and 97.5% percentiles of the posterior distributions. The posterior probability of association (POA) in the direction of the median is also presented. All of the statistical analyses we performed with the R ver. 4.0.5 and Stan (CmdStan ver. 2.30.1) software packages [34, 35].
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Results
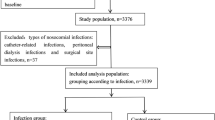
In total, 333 patients who were diagnosed with SLE based on the 1982 ACR and/or the 2012 SLICC classification criteria were included in the analyses [17, 18]. The majority of the cases were women (86%) and more than 88% of the patients were of Caucasian ethnicity/White race. Altogether, during the study period (2008‒2022), the patients were monitored at 3,088 visits to the rheumatologist. The characteristics of the included patients are listed in Table 1.
Episodes of neutropenia
We identified 94 episodes of neutropenia in 40 patients (12% of all included cases). Among these patients, the proportion of women was 90%. The average age at which neutropenic episodes occurred was 44.4 years (SD, 13). In 88/94 (94%) of the neutropenic episodes, combined ongoing hematologic manifestations (58 leukopenia, 41 lymphopenia, 36 anemia and 16 thrombocytopenia) were observed. Thus, only six episodes of isolated neutropenia were found. The mean NLR of the 94 neutropenic episodes was 1.03 (SD, 0.65). The most commonly detected autoantibody specificity in patients with neutropenic episodes was anti-SSA (Ro60 and/or Ro52/TRIM21), which was found in 62% of the neutropenic patients (42% of the overall study population). Details concerning the characteristics of the patients with episodes of neutropenia and ongoing treatments during neutropenic episodes are provided in Table 2.
Infections in patients with or without neutropenia
During the study period, a total of 918 infections was identified among 237 patients (71%). Regarding those patients who had at least one infection, 85% were women. The average age of the patients who experienced infections was 56.2 years (SD, 18.2).
Among the 918 infections, 30 (3.3%) co-occurred with confirmed neutropenia, clustered among 15 patients. Details regarding ongoing immunosuppressive therapy, daily glucocorticoid dose, NLR and the infecting pathogens are provided in Supplementary Table 1. The NLR among patients with higher severity of infection (level 3 or 4; mean 0.95, SD, 0.67) was not significantly different than in those with less severity of infection (level 1 or 2; mean 0.74, SD, 0.57). Both patients with agranulocytosis (Supplementary Table 1) received granulocyte–macrophage colony-stimulating factor (GM-CSF).
The remaining 888 infections could be divided into 274 infections (in 131 patients) without neutropenia and 614 infections (in 211 patients) in which WBC differentiation had not been assessed in conjunction with the infections (note that infections may have occurred more than once per patient, regardless of the ANC). Throughout the study period, the odds of infections were higher among individuals who (on any occasion) had experienced neutropenia than among those who had no confirmed neutropenic episodes (OR = 2.09, 95% CI 0.94;5.19, POA = 96.3%). The characteristics of the infections are presented in Table 3, divided according to the three neutropenia-related groups.
Neutropenia combined with hypocomplementemia and/or lymphopenia
The proportion of neutropenic episodes that occurred in patients meeting the SLICC ‘low complement’ criterion was 21.3%, and there was no strong evidence that the odds of infection between the groups were different (OR = 2.1, 95% CI 0.24;24.15; POA = 75.9%). The proportions of neutropenic episodes that occurred or did not occur in patients with meeting the SLICC ‘lymphopenia’ criterion were similar, and the estimated odds of infection were also similar between the groups (OR = 1.04, 95% CI 0.26;4.36, POA = 52.2%). The characteristics of the infections with or without hypocomplementemia and/or lymphopenia are listed in Table 4.
Neutropenia, lymphopenia, and hypocomplementemia in relation to the invasiveness and severity of infections
Table 5 shows the estimated conditional ORs describing the associations between the invasiveness and severity of infections and confirmed neutropenia, confirmed absence of neutropenia, or not controlled. As shown, the odds for an invasive infection were higher in subjects who had confirmed neutropenia versus those who did not. The odds of having a severe infection were also higher in those subjects who had confirmed neutropenia versus those who did not. The regression models were adjusted for variables that represented whether or not patients had ongoing or a history of hypocomplementemia or lymphopenia during the study period, and the interaction between the two items. The coefficients for these covariates indicated that patients who had at least one documented episode of hypocomplementemia were less likely to have invasive infections than patients who had had no documented episodes of hypocomplementemia, conditional on the other covariates in the model. Patients who had at least one documented episode of lymphopenia over the study period were more likely to have higher severity of infections compared to those who had no documented episodes of lymphopenia, conditional on the other covariates in the model. Finally, patients who had at least one documented episode of hypocomplementemia and lymphopenia were less likely to have higher severity infections compared to those who had no documented episodes of either.
Pathogens
Notably, we identified a high number of infections, managed in the outpatient care setting, which had not been further investigated using microbiologic analyses. Altogether, any microbiological analysis (cultures of blood, urine, sputum or nasopharyngeal swab were most common) was ordered in 437/918 infections (47.6%), but 98 of these analyses fell out negative. Thus, support for infection by any microbiologic analysis (i.e., culture, serology, or viral detection) was available in 339/918 infections (36.9%). The pathogens responsible for infections in the 30 cases with neutropenia are detailed in Supplementary Table 1. Of note, in only 2/30 infections (6.7%) were the patients entirely off glucocorticoid treatment, and opportunistic infections with Pneumocystis jirovecii were seen in two individuals who were receiving high daily doses of prednisolone. Overall, the most frequently detected bacteria causing infections were pathogens affecting the respiratory tract, urinary tract, and skin. Escherichia coli and Enterococcus faecalis, Streptococcus pneumoniae, Haemophilus influenzae, Klebsiella pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa were the predominant bacteria in the cultures of patients. Viral infections were caused by herpes simplex virus and varicella zoster virus, cytomegalovirus and/or Epstein–Barr virus, and SARS-CoV-2. Fungal infections were predominantly caused by Candida species at different locations in the body.
Discussion
Herein, we investigated the prevalence of neutropenia and the associations with confirmed infections in well-characterized Swedish subjects with SLE who were residing in Östergötland County over a 14-year time period [30]. We show that 12% of the patients experienced at least one episode of (usually, mild) neutropenia over time. In general, infections were common among the study population, but only a minority of the infections were associated with neutropenia. Nevertheless, both the invasiveness and severity of the infections were significantly associated with neutropenia, whereas ongoing or a history of lymphopenia and/or hypocomplementemia did not affect the association to the same extent.
Although the KLURING cohort contains mainly patients of Caucasian ethnicity, our findings are similar to those recently published for the Toronto Lupus Cohort, in which isolated neutropenia was associated with Black race [36]. A systematic review from 2015 reported a slightly higher prevalence (range 20%‒40%) of neutropenia in patients with SLE [37]. It cannot be excluded that the divergent results are related to differences in SLE phenotypes, ethnicities, and/or the selection of overall more severely affected patients with more-severe SLE requiring more or higher doses of immunosuppressive agents [38]. In line with the observations made by others, we found that approximately two-thirds of all the episodes of neutropenia in SLE were mild (ANC < 1.5 × 109/L ‒ ≥ 1.0 × 109/L) [25]. Furthermore, we observed that neutropenia was usually accompanied by other hematologic abnormalities, such as anemia, leukopenia and lymphopenia, as well as the presence of anti-SSA antibodies (62%). The latter has also been observed by others, and Kurien et al. suggested that anti-SSA antibodies are directly involved in mediating neutropenia in patients with SLE [39].
The use of immunosuppressive agents is an obvious independent risk factor for neutropenia due to drug toxicity-induced medullary hypoplasia [25]. In addition, the use of rituximab in patients with SLE has been associated with late-onset neutropenia [26]. However, treatment with glucocorticoids (corresponding to a daily dose of 15 mg prednisone) may lead to a significantly increased number of neutrophils in the circulation [40]. In the present study, 62% of the patients used prednisone dosages ≤ 5 mg when the neutropenia was identified, and 37% used immunosuppressive agents. This indicates that most of the neutropenic episodes detected in this study are more likely part of the SLE pathogenesis per se than the side effects of immunosuppression, as mentioned previously [41]. Obviously, high doses of glucocorticoids have multiple effects on other blood cells; e.g., circulating lymphocyte levels may decrease, but could also be a result of SLE with different degree of severity [19]. NLR is frequently used as a prognostic marker in critically ill patients with infections [42]. In SLE, some studies have found elevated NLR useful to distinguish infections from flares whereas other claim that high NLR rather may be associated to active SLE, activation of the classical complement pathway, advanced organ damage, severe depression, and poor quality of life [43,44,45,46]. Our study did not observe any statistically significant difference in NLR between neutropenic patients with mild and severe infection.
Neutrophils play essential roles in host immune defenses, as they ingest, kill, and digest invading microorganisms. Interestingly, new data indicate that neutrophils may also play an important role in the resolution of inflammation [47]. However, this phenomenon has not yet been studied in detail in SLE. Although neutropenia is a far less common hematologic manifestation of SLE than lymphopenia, the overall interest in neutrophils in the context of autoimmunity, and particularly SLE, has grown significantly over the last decade. Impaired handling of apoptotic cells with exposure of modified self-antigens from dying cells, including NETs in a proinflammatory environment, affects immune tolerance with subsequent development of autoimmunity and deterioration of already established autoimmune disease [29, 48]. Exposure to the immune system of otherwise hidden molecules, such as DNA and nuclear constituents, logically represents a starting step for autoimmunity, and in SLE and antiphospholipid syndrome (APS), a functional neutrophil is important for preventing loss of tolerance via immune regulation and clearance [41, 49,50,51]. In support of this, NETs have been shown to contribute to inflammation and tissue damage in a number of other organs in patients with SLE, such as the brain, heart, and lung [52,53,54].
In the current study, approximately 80% of the infections observed were deemed to be bacterial, and they mainly affected the respiratory and urogenital tracts, skin, bone and soft tissues. Similar findings have previously been reported by the Spanish Rheumatology Society Lupus Registry regarding severe infections and bacteremia [13, 14]. Only a minority of the infections in the present cohort were severe and, thus the majority were managed with outpatient care regardless of neutropenia, probably reflecting effective health controls and access to the public healthcare system in this region of Sweden. Consequently, neutrophil counts were not assessed in conjunction with the majority of the infections. As a result, cultures and serologic tests for different causal microorganisms were not performed for > 50% of the infections, as they were managed as outpatients or were mild cases.
When the patients were divided based on ANC, to compare the infections, higher odds of severe infections were noted for patients with neutropenia, as compared with those patients who did not have neutropenia. In addition, the odds of developing invasive infections were also higher for subjects with confirmed neutropenia in our study. In agreement with a recent report, we did not find any evidence that lymphopenia aggravate the odds of infection [36]. A meta-analysis focusing on characteristics and risk factors of infection in SLE showed that after, adjusting for several factors, lymphopenia was a less important risk factor for infection whereas continuous complement consumption appeared to be worse [55].
There are some limitations to the present study that need to be considered when interpreting our findings. For this observational study, we did not include any control subjects with other diseases, and we did not have enough statistical power to evaluate any influence of immunosuppressive therapies. In addition, the relatively few neutropenic episodes in the dataset make it difficult to estimate the odds ratio with high precision and causes of neutropenia were not investigated (e.g., drug-induced neutropenia). Moreover, most of the cases included were outpatients for whom WBC differentiation tests had not been performed; thus, it is possible that some mild neutropenic episodes might have been missed. Nevertheless, the study is a monocenter cohort study with a well-controlled study population that was followed systematically over a 14-year period. Finally, it should be emphasized that the Swedish healthcare system is public, tax funded, and offers universal access, which constitute major strengths.
In conclusion, infections are common in patients with SLE and occur in both the presence and absence of neutropenia. Nevertheless, confirmed neutropenia co-appearing with infections seems to be associated with both the invasiveness and severity of infections, whereas a history of lymphopenia and/or hypocomplementemia did not seem to be as important in this context. Our results have implications for healthcare institutions in suggesting that they perform WBC differentiation testing as part of the risk stratification for patients with SLE who show signs of infection. Since infections still constitute a leading cause of mortality in SLE, neutropenia is relevant and important to consider in patients who have infections, to prevent severe illness and death.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, Ruiz-Irastorza G, Hughes G (2016) Systemic lupus erythematosus. Nat Rev Dis Primers 2:16039. https://doi.org/10.1038/nrdp.2016.39
Björk M, Dahlström Ö, Wetterö J, Sjöwall C (2015) Quality of life and acquired organ damage are intimately related to activity limitations in patients with systemic lupus erythematosus. BMC Musculoskelet Disord 16:188. https://doi.org/10.1186/s12891-015-0621-3
Frodlund M, Jönsen A, Remkus L, Telg G, Söderdahl F, Leonard D (2023) Glucocorticoid treatment in SLE is associated with infections, comorbidities, and mortality-a national cohort study. Rheumatology. https://doi.org/10.1093/rheumatology/kead348
Ugarte-Gil MF, Mak A, Leong J, Dharmadhikari B, Kow NY, Reátegui-Sokolova C, Elera-Fitzcarrald C, Aranow C, Arnaud L, Askanase AD, Bae SC, Bernatsky S, Bruce IN, Buyon J, Costedoat-Chalumeau N, Dooley MA, Fortin PR, Ginzler EM, Gladman DD, Hanly J, Inanc M, Isenberg D, Jacobsen S, James JA, Jönsen A, Kalunian K, Kamen DL, Lim SS, Morand E, Mosca M, Peschken C, Pons-Estel BA, Rahman A, Ramsey-Goldman R, Reynolds J, Romero-Diaz J, Ruiz-Irastorza G, Sánchez-Guerrero J, Svenungsson E, Urowitz M, Vinet E, van Vollenhoven RF, Voskuyl A, Wallace DJ, Petri MA, Manzi S, Clarke AE, Cheung M, Farewell V, Alarcon GS (2021) Impact of glucocorticoids on the incidence of lupus-related major organ damage: a systematic literature review and meta-regression analysis of longitudinal observational studies. Lupus Sci Med. https://doi.org/10.1136/lupus-2021-000590
Danza A, Ruiz-Irastorza G (2013) Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus 22(12):1286–1294. https://doi.org/10.1177/0961203313493032
Frodlund M, Nived P, Chatzidionysiou A, Södergren A, Klingberg E, Bengtsson A, Hansson M, Olsson S, Pin E, Klareskog L, Kapetanovic MC (2023) The impact of immunomodulating treatment on the immunogenicity of COVID-19 vaccines in patients with immune-mediated inflammatory rheumatic diseases compared to healthy controls. A Swedish nationwide study (COVID19-REUMA). Vaccine 41(20):3247–3257. https://doi.org/10.1016/j.vaccine.2023.03.065
Parodis I, Stockfelt M, Sjöwall C (2020) B cell therapy in systemic lupus erythematosus: from rationale to clinical practice. Front Med (Lausanne) 7:316. https://doi.org/10.3389/fmed.2020.00316
Sjöwall J, Azharuddin M, Frodlund M, Zhang Y, Sandner L, Dahle C, Hinkula J, Sjöwall C (2021) SARS-CoV-2 antibody isotypes in systemic lupus erythematosus patients prior to vaccination: associations with disease activity, antinuclear antibodies, and immunomodulatory drugs during the first year of the pandemic. Front Immunol 12:724047. https://doi.org/10.3389/fimmu.2021.724047
Edwards CJ, Lian TY, Badsha H, Teh CL, Arden N, Chng HH (2003) Hospitalization of individuals with systemic lupus erythematosus: characteristics and predictors of outcome. Lupus 12(9):672–676. https://doi.org/10.1191/0961203303lu452oa
Goldblatt F, Chambers S, Rahman A, Isenberg DA (2009) Serious infections in British patients with systemic lupus erythematosus: hospitalisations and mortality. Lupus 18(8):682–689. https://doi.org/10.1177/0961203308101019
Petri M, Genovese M (1992) Incidence of and risk factors for hospitalizations in systemic lupus erythematosus: a prospective study of the Hopkins Lupus Cohort. J Rheumatol 19(10):1559–1565
Simard JF, Rossides M, Gunnarsson I, Svenungsson E, Arkema EV (2021) Infection hospitalisation in systemic lupus in Sweden. Lupus Sci Med. https://doi.org/10.1136/lupus-2021-000510
Rúa-Figueroa Í, López-Longo J, Galindo-Izquierdo M, Calvo-Alén J, Del Campo V, Olivé-Marqués A, Pérez-Vicente S, Fernández-Nebro A, Andrés M, Erausquin C, Tomero E, Horcada L, Uriarte E, Freire M, Montilla C, Sánchez-Atrio A, Santos G, Boteanu A, Díez-Álvarez E, Narváez J, Martínez-Taboada V, Silva-Fernández L, Ruiz-Lucea E, Andreu JL, Hernández-Beriain J, Gantes M, Hernández-Cruz B, Pérez-Venegas J, Pecondón-Español Á, Marras C, Ibáñez-Barceló M, Bonilla G, Torrente V, Castellví I, Alegre JJ, Calvet J, Marenco JL, Raya E, Vázquez T, Quevedo V, Muñoz-Fernández S, Rodríguez-Gómez M, Ibáñez J, Pego-Reigosa JM (2017) Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum 47(1):38–45. https://doi.org/10.1016/j.semarthrit.2017.01.010
Rúa-Figueroa I, López-Longo FJ, Del Campo V, Galindo-Izquierdo M, Uriarte E, Torre-Cisneros J, Vela P, Tomero E, Narváez J, Olivé A, Freire M, Salgado E, Andreu JL, Martínez-Taboada V, Calvo-Alén J, Hernández-Cruz B, Raya E, Quevedo V, Expósito Pérez L, Fernández-Nebro A, Ibañez M, Pascual-Valls È, Rúa-Figueroa D, Naranjo A, Pego-Reigosa JM (2020) Bacteremia in systemic lupus erythematosus in patients from a spanish registry: risk factors, clinical and microbiological characteristics, and outcomes. J Rheumatol 47(2):234–240. https://doi.org/10.3899/jrheum.180882
Battaglia M, Garrett-Sinha LA (2021) Bacterial infections in lupus: roles in promoting immune activation and in pathogenesis of the disease. J Transl Autoimmun 4:100078. https://doi.org/10.1016/j.jtauto.2020.100078
Zandman-Goddard G, Shoenfeld Y (2005) Infections and SLE. Autoimmunity 38(7):473–485. https://doi.org/10.1080/08916930500285352
Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sanchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G Jr, Magder LS (2012) Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64(8):2677–2686. https://doi.org/10.1002/art.34473
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25(11):1271–1277. https://doi.org/10.1002/art.1780251101
Yavuz S, Cansu DU, Nikolopoulos D, Crisafulli F, Antunes AM, Adamichou C, Reid S, Stagnaro C, Andreoli L, Tincani A, Moraes-Fontes MF, Mosca M, Leonard D, Jönsen A, Bengtsson A, Svenungsson E, Gunnarsson I, Dahlqvist SR, Sjöwall C, Bertsias G, Fanouriakis A, Rönnblom L (2020) Lymphopenia as a risk factor for neurologic involvement and organ damage accrual in patients with systemic lupus erythematosus: a multi-center observational study. Semin Arthritis Rheum 50(6):1387–1393. https://doi.org/10.1016/j.semarthrit.2020.02.020
Bacon BR, Treuhaft WH, Goodman AM (1981) Azathioprine-induced pancytopenia. Occurrence in two patients with connective-tissue diseases. Arch Intern Med 141(2):223–226
Rosenthal NS, Farhi DC (1989) Bone marrow findings in connective tissue disease. Am J Clin Pathol 92(5):650–654. https://doi.org/10.1093/ajcp/92.5.650
Meyer A, Guffroy A, Blaison G, Dieudonne Y, Amoura Z, Bonnotte B, Fiehn C, Kieffer P, Lorenz HM, Magy-Bertrand N, Maurier F, Pennaforte JL, Peter HH, Schwarting A, Sibilia J, Arnaud L, Martin T, Voll RE, Korganow AS (2020) Systemic lupus erythematosus and neutropaenia: a hallmark of haematological manifestations. Lupus Sci Med. https://doi.org/10.1136/lupus-2020-000399
Rustagi PK, Currie MS, Logue GL (1985) Complement-activating antineutrophil antibody in systemic lupus erythematosus. Am J Med 78(6 Pt 1):971–977. https://doi.org/10.1016/0002-9343(85)90220-7
Boxer LA, Greenberg MS, Boxer GJ, Stossel TP (1975) Autoimmune neutropenia. N Engl J Med 293(15):748–753. https://doi.org/10.1056/nejm197510092931505
Martínez-Baños D, Crispín JC, Lazo-Langner A, Sánchez-Guerrero J (2006) Moderate and severe neutropenia in patients with systemic lupus erythematosus. Rheumatology (Oxford) 45(8):994–998. https://doi.org/10.1093/rheumatology/kel016
Parodis I, Söder F, Faustini F, Kasza Z, Samuelsson I, Zickert A, Svenungsson E, van Vollenhoven RF, Malmström V, Wermeling F, Gunnarsson I (2018) Rituximab-mediated late-onset neutropenia in systemic lupus erythematosus - distinct roles of BAFF and APRIL. Lupus 27(9):1470–1478. https://doi.org/10.1177/0961203318777116
Campion G, Maddison PJ, Goulding N, James I, Ahern MJ, Watt I, Sansom D (1990) The Felty syndrome: a case-matched study of clinical manifestations and outcome, serologic features, and immunogenetic associations. Medicine (Baltimore) 69(2):69–80
Hellmich B, Csernok E, de Haas M, von dem Borne AE, Schatz H, Gross WL, Schnabel A (2002) Low Fcgamma receptor III and high granulocyte colony-stimulating factor serum levels correlate with the risk of infection in neutropenia due to Felty’s syndrome or systemic lupus erythematosus. Am J Med 113(2):134–139. https://doi.org/10.1016/s0002-9343(02)01161-0
Urbonaviciute V, Luo H, Sjöwall C, Bengtsson A, Holmdahl R (2019) Low production of reactive oxygen species drives systemic lupus erythematosus. Trends Mol Med 25(10):826–835. https://doi.org/10.1016/j.molmed.2019.06.001
Arkema EV, Saleh M, Simard JF, Sjöwall C (2023) Epidemiology and damage accrual of systemic lupus erythematosus in central sweden: a single-center population-based cohort study over 14 years from Östergötland county. ACR Open Rheumatol 5(8):426–432. https://doi.org/10.1002/acr2.11585
Boxer LA (2012) How to approach neutropenia. Hematol Am Soc Hematol Educ Progr 2012:174–182. https://doi.org/10.1182/asheducation-2012.1.174
Valent P (2012) Low blood counts: immune mediated, idiopathic, or myelodysplasia. Hematol Am Soc Hematol Educ Progr 2012:485–491. https://doi.org/10.1182/asheducation-2012.1.485
Bendtsen M (2018) A gentle introduction to the comparison between null hypothesis testing and bayesian analysis: reanalysis of two randomized controlled trials. J Med Internet Res 20(10):e10873. https://doi.org/10.2196/10873
Team RC (2020) R: a language and environment for statistical computing. r foundation for statistical computing. https://www.r-project.org/
Team SD (2022) Stan modeling language users guide and reference manual, 2.30.1. https://mc-stan.org
Alhammadi NA, Gladman DD, Su J, Urowitz MB (2023) Isolated neutropenia in systemic lupus erythematosus. J Rheumatol 50(3):459–460. https://doi.org/10.3899/jrheum.220373
Carli L, Tani C, Vagnani S, Signorini V, Mosca M (2015) Leukopenia, lymphopenia, and neutropenia in systemic lupus erythematosus: prevalence and clinical impact–A systematic literature review. Semin Arthritis Rheum 45(2):190–194. https://doi.org/10.1016/j.semarthrit.2015.05.009
Maningding E, Dall’Era M, Trupin L, Murphy LB, Yazdany J (2020) Racial and ethnic differences in the prevalence and time to onset of manifestations of systemic lupus erythematosus: the california lupus surveillance project. Arthritis Care Res 72(5):622–629. https://doi.org/10.1002/acr.23887
Kurien BT, Newland J, Paczkowski C, Moore KL, Scofield RH (2000) Association of neutropenia in systemic lupus erythematosus (SLE) with anti-Ro and binding of an immunologically cross-reactive neutrophil membrane antigen. Clin Exp Immunol 120(1):209–217. https://doi.org/10.1046/j.1365-2249.2000.01195.x
Sugimoto T, Soumura M, Tanaka Y, Uzu T, Nishio Y, Kashiwagi A (2006) Early morning neutropenia in a patient with systemic lupus erythematosus. Mod Rheumatol 16(4):267–268. https://doi.org/10.1007/s10165-006-0483-5
Wirestam L, Arve S, Linge P, Bengtsson AA (2019) Neutrophils-important communicators in systemic lupus erythematosus and antiphospholipid syndrome. Front Immunol 10:2734. https://doi.org/10.3389/fimmu.2019.02734
Li X, Liu C, Mao Z, Xiao M, Wang L, Qi S, Zhou F (2020) Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care 24(1):647. https://doi.org/10.1186/s13054-020-03374-8
Li Z, Xiao Y, Zhang L (2020) Application of procalcitonin, white blood cell count and neutrophil-to-lymphocyte ratio in the diagnosis of systemic lupus erythematosus with a bacterial infection. Ann Palliat Med 9(6):3870–3876. https://doi.org/10.21037/apm-20-1777
Mercader-Salvans J, García-González M, Quevedo-Abeledo JC, Quevedo-Rodríguez A, Romo-Cordero A, Ojeda-Bruno S, Gómez-Bernal F, López-Mejías R, Martín-González C, González-Gay M, Ferraz-Amaro I (2023) Blood Composite Scores in Patients with Systemic Lupus Erythematosus. Biomedicines. https://doi.org/10.3390/biomedicines11102782
Musunuri B, Tripathy R, Padhi S, Panda AK, Das BK (2022) The role of MBL, PCT, CRP, neutrophil-lymphocyte ratio, and platelet lymphocyte ratio in differentiating infections from flares in lupus. Clin Rheumatol 41(11):3337–3344. https://doi.org/10.1007/s10067-022-06285-x
Papachristodoulou E, Kakoullis L, Christophi C, Psarelis S, Hajiroussos V, Parperis K (2023) The relationship of neutrophil-to-lymphocyte ratio with health-related quality of life, depression, and disease activity in SLE: a cross-sectional study. Rheumatol Int 43(10):1841–1848. https://doi.org/10.1007/s00296-023-05381-8
Filep JG, Ariel A (2020) Neutrophil heterogeneity and fate in inflamed tissues: implications for the resolution of inflammation. Am J Physiol Cell Physiol 319(3):C510-c532. https://doi.org/10.1152/ajpcell.00181.2020
Bruschi M, Moroni G, Sinico RA, Franceschini F, Fredi M, Vaglio A, Cavagna L, Petretto A, Pratesi F, Migliorini P, Manfredi A, Ramirez GA, Esposito P, Negrini S, Trezzi B, Emmi G, Santoro D, Scolari F, Volpi S, Mosca M, Tincani A, Candiano G, Prunotto M, Verrina E, Angeletti A, Ravelli A, Ghiggeri GM (2021) Neutrophil extracellular traps in the autoimmunity context. Front Med (Lausanne) 8:614829. https://doi.org/10.3389/fmed.2021.614829
Bruschi M, Petretto A, Santucci L, Vaglio A, Pratesi F, Migliorini P, Bertelli R, Lavarello C, Bartolucci M, Candiano G, Prunotto M, Ghiggeri GM (2019) Neutrophil Extracellular Traps protein composition is specific for patients with Lupus nephritis and includes methyl-oxidized αenolase (methionine sulfoxide 93). Sci Rep 9(1):7934. https://doi.org/10.1038/s41598-019-44379-w
Knopf J, Sjöwall J, Frodlund M, Hinkula J, Herrmann M, Sjöwall C (2022) NET formation in systemic lupus erythematosus: changes during the COVID-19 pandemic. Cells. https://doi.org/10.3390/cells11172619
Li Y, Wu Y, Huang J, Cao X, An Q, Peng Y, Zhao Y, Luo Y (2023) A variety of death modes of neutrophils and their role in the etiology of autoimmune diseases. Immunol Rev. https://doi.org/10.1111/imr.13284
Allen C, Thornton P, Denes A, McColl BW, Pierozynski A, Monestier M, Pinteaux E, Rothwell NJ, Allan SM (2012) Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA. J Immunol 189(1):381–392. https://doi.org/10.4049/jimmunol.1200409
Appelgren D, Dahle C, Knopf J, Bilyy R, Vovk V, Sundgren PC, Bengtsson AA, Wetterö J, Muñoz LE, Herrmann M, Höög A, Sjöwall C (2018) Active NET formation in Libman-Sacks endocarditis without antiphospholipid antibodies: a dramatic onset of systemic lupus erythematosus. Autoimmunity 51(6):310–318. https://doi.org/10.1080/08916934.2018.1514496
Jarrot PA, Tellier E, Plantureux L, Crescence L, Robert S, Chareyre C, Daniel L, Secq V, Garcia S, Dignat-George F, Panicot-Dubois L, Dubois C, Kaplanski G (2019) Neutrophil extracellular traps are associated with the pathogenesis of diffuse alveolar hemorrhage in murine lupus. J Autoimmun 100:120–130. https://doi.org/10.1016/j.jaut.2019.03.009
Yuan Q, Xing X, Lu Z, Li X (2020) Clinical characteristics and risk factors of infection in patients with systemic lupus erythematosus: a systematic review and meta-analysis of observational studies. Semin Arthritis Rheum 50(5):1022–1039. https://doi.org/10.1016/j.semarthrit.2020.06.004
Acknowledgements
We are sincerely grateful to all the participating patients, and we thank all the clinicians at the Rheumatology Clinic, Linköping University Hospital, for their collaboration.
Funding
Open access funding provided by Linköping University. This work was supported by grants from the Swedish Rheumatism Association, the Region Östergötland (ALF Grants), the King Gustaf V’s 80-year Anniversary Foundation, the King Gustaf V and Queen Victoria’s Freemasons Foundations, and the Ulla and Roland Gustafsson Foundation.
Author information
Authors and Affiliations
Contributions
All authors included in the paper fulfilled the criteria of authorship. MS, JS, CS: study design, data collection, statistical analyses, manuscript writing, and final approval; MB: statistical analyses, manuscript writing, and final approval; CS: supervision.
Corresponding author
Ethics declarations
Conflict of interest
The other authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
Oral and written informed consent was obtained from all the included patients in the KLURING registry. The study protocol was approved by the Regional Ethics Review Board in Linköping (Decision no. M75-08, May 21st 2008, Linköping).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Congress abstract publication: This study was accepted as a plenary session presentation at the Scandinavian Congress of Rheumatology in Copenhagen, Denmark (2023).
Saleh M, Sjöwall J, Bendtsen M, Sjöwall C. OP04: Prevalence of neutropenia and its association to infections in patients with systemic lupus erythematosus: A single-centre cohort study over 14 years. Scand J Rheumatol. 2023;52(Suppl.131):11-12 https://www-tandfonline-com.e.bibl.liu.se/doi/full/10.1080/03009742.2023.2233370.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saleh, M., Sjöwall, J., Bendtsen, M. et al. The prevalence of neutropenia and association with infections in patients with systemic lupus erythematosus: a Swedish single-center study conducted over 14 years. Rheumatol Int 44, 839–849 (2024). https://doi.org/10.1007/s00296-024-05566-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-024-05566-9