Abstract
Background
Quantitative magnetic resonance imaging (MRI) could improve the estimation of fetal brain maturation and the interpretation of white matter signal intensity in pathological conditions.
Objective
To investigate T2-based and diffusion-weighted imaging (DWI) measurements for the evaluation of fetal brain maturation during the last trimester of pregnancy.
Materials and methods
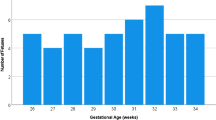
One hundred sixty-eight fetal brain MRIs were retrospectively analyzed (age range: 28–37 weeks of gestation) after ensuring that none of the children developed psychomotor or cognitive impairment (median follow-up: 4.7 years). Bilateral regions of interest were drawn on the frontal, occipital, parietal and temporal lobes from T2-W imaging and DWI, when available, to evaluate signal intensity and apparent diffusion coefficient (ADC) values. Ratios were calculated with two references (pons or thalamus and cerebrospinal fluid) to standardize signal intensities. Reproducibility was evaluated with intraclass correlation coefficients (ICCs) and Bland-Altman plots. Correlations with gestational age were evaluated with univariate and multivariate linear regressions.
Results
T2 measurements were achieved in all cases, and DWI was available in 37 cases. Measurements and ratios were reproducible in eight localizations (i.e. intra- and interobserver ICCs >0.5): frontal T2/thalamus, parietal T2/thalamus, occipital T2/pons, parietal ADC/thalamus, occipital ADC/pons, temporal ADC/pons, occipital ADC and temporal ADC. The frontal T2/thalamus and parietal T2/thalamus correlated with gestational age (P<0.0001 and P=0.014, respectively). In the multivariate modeling, frontal T2/thalamus remained an independent predictor of the gestational age (P<0.0001).
Conclusion
The frontal T2/thalamus ratio emerged as a potential additional biomarker of fetal brain maturation during the last trimester of pregnancy.







Similar content being viewed by others
References
Diogo MC, Glatter S, Binder J et al (2020) The MRI spectrum of congenital cytomegalovirus infection. Prenat Diagn 40:110–124
Lipitz S, Hoffmann C, Feldman B et al (2010) Value of prenatal ultrasound and magnetic resonance imaging in assessment of congenital primary cytomegalovirus infection. Ultrasound Obstet Gynecol 36:709–717
Garel C (2004) The role of MRI in the evaluation of the fetal brain with an emphasis on biometry, gyration and parenchyma. Pediatr Radiol 34:694–699
Averill LW, Kandula VVR, Akyol Y, Epelman M (2015) Fetal brain magnetic resonance imaging findings in congenital cytomegalovirus infection with postnatal imaging correlation. Semin Ultrasound CT MR 36:476–486
Dangouloff-Ros V, Roux C-J, Boulouis G et al (2019) Incidental brain MRI findings in children: a systematic review and meta-analysis. AJNR Am J Neuroradiol 40:1818–1823
Kristjnsdóttir R, Uvebrant P, Wiklund LM (2000) Clinical characteristics of children with cerebral white matter abnormalities. Eur J Paediatr Neurol 4:17–26
Fazekas F, Kleinert R, Offenbacher H et al (1993) Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43:1683–1689
Katorza E, Strauss G, Cohen R et al (2018) Apparent diffusion coefficient levels and neurodevelopmental outcome in fetuses with brain MR imaging white matter hyperintense signal. AJNR Am J Neuroradiol 39:1926–1931
Neuberger I, Garcia J, Meyers ML et al (2018) Imaging of congenital central nervous system infections. Pediatr Radiol 48:513–523
Garel C (2006) New advances in fetal MR neuroimaging. Pediatr Radiol 36:621–625
Hoffmann C, Weisz B, Lipitz S et al (2014) Regional apparent diffusion coefficient values in 3rd trimester fetal brain. Neuroradiology 56:561–567
Sartor A, Arthurs O, Alberti C et al (2014) Apparent diffusion coefficient measurements of the fetal brain during the third trimester of pregnancy: how reliable are they in clinical practice? Prenat Diagn 34:357–366
Schneider JF, Confort-Gouny S, Le Fur Y et al (2007) Diffusion-weighted imaging in normal fetal brain maturation. Eur Radiol 17:2422–2429
Boyer AC, Gonçalves LF, Lee W et al (2013) Magnetic resonance diffusion-weighted imaging: reproducibility of regional apparent diffusion coefficients for the normal fetal brain. Ultrasound Obstet Gynecol 41:190–197
Schneider MM, Berman JI, Baumer FM et al (2009) Normative apparent diffusion coefficient values in the developing fetal brain. AJNR Am J Neuroradiol 30:1799–1803
Cannie M, De Keyzer F, Meersschaert J et al (2007) A diffusion-weighted template for gestational age-related apparent diffusion coefficient values in the developing fetal brain. Ultrasound Obstet Gynecol 30:318–324
Stazzone MM, Hubbard AM, Bilaniuk LT et al (2000) Ultrafast MR imaging of the normal posterior fossa in fetuses. AJR Am J Roentgenol 175:835–839
Huisman TAGM, Martin E, Kubik-Huch R, Marincek B (2002) Fetal magnetic resonance imaging of the brain: technical considerations and normal brain development. Eur Radiol 12:1941–1951
Abe S, Takagi K, Yamamoto T et al (2004) Semiquantitative assessment of myelination using magnetic resonance imaging in normal fetal brains. Prenat Diagn 24:352–357
Leppert IR, Almli CR, McKinstry RC et al (2009) T(2) relaxometry of normal pediatric brain development. J Magn Reson Imaging 29:258–267
Counsell SJ, Kennea NL, Herlihy AH et al (2003) T2 relaxation values in the developing preterm brain. AJNR Am J Neuroradiol 24:1654–1660
Thornton JS, Amess PN, Penrice J et al (1999) Cerebral tissue water spin-spin relaxation times in human neonates at 2.4 tesla: methodology and the effects of maturation. Magn Reson Imaging 17:1289–1295
Wickham H, Averick M, Bryan J et al (2019) Welcome to the Tidyverse. J Open Source Softw 4:1686
Vovk U, Pernus F, Likar B A review of methods for correction of intensity inhomogeneity in MRI. IEEE Trans Med Imaging 26:405–421
Sun X, Shi L, Luo Y et al (2015) Histogram-based normalization technique on human brain magnetic resonance images from different acquisitions. Biomed Eng Online 14:73
Fortin J-P, Sweeney EM, Muschelli J et al (2016) Removing inter-subject technical variability in magnetic resonance imaging studies. Neuroimage 132:198–212
De Nunzio G, Cataldo R, Carlà A (2015) Robust intensity standardization in brain magnetic resonance images. J Digit Imaging 28:727–737
Yu M, Linn KA, Cook PA et al (2018) Statistical harmonization corrects site effects in functional connectivity measurements from multi-site fMRI data. Hum Brain Mapp 39:4213–4227
Leroy F, Glasel H, Dubois J et al (2011) Early maturation of the linguistic dorsal pathway in human infants. J Neurosci 31:1500–1506
Glasser MF, Van Essen DC (2011) Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci 31:11597–11616
Bui T, Daire J-L, Chalard F et al (2006) Microstructural development of human brain assessed in utero by diffusion tensor imaging. Pediatr Radiol 36:1133–1140
Righini A, Bianchini E, Parazzini C et al (2003) Apparent diffusion coefficient determination in normal fetal brain: a prenatal MR imaging study. AJNR Am J Neuroradiol 24:799–804
Autti T, Raininko R, Vanhanen SL et al (1994) MRI of the normal brain from early childhood to middle age. I. Appearances on T2- and proton density-weighted images and occurrence of incidental high-signal foci. Neuroradiology 36:644–648
Luoma K, Raininko R, Nummi P, Luukkonen R (1993) Is the signal intensity of cerebrospinal fluid constant? Intensity measurements with high and low field magnetic resonance imagers. Magn Reson Imaging 11:549–555
Cartry C, Viallon V, Hornoy P, Adamsbaum C (2010) Diffusion-weighted MR imaging of the normal fetal brain: marker of fetal brain maturation. J Radiol 91(5 Pt 1):561–566
Han R, Huang L, Sun Z et al (2015) Assessment of apparent diffusion coefficient of normal fetal brain development from gestational age week 24 up to term age: a preliminary study. Fetal Diagn Ther 37:102–107
Barkovich AJ, Kjos BO, Jackson DE, Norman D (1988) Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology 166:173–180
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927
Gao W, Lin W, Chen Y et al (2009) Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR Am J Neuroradiol 30:290–296
Hasegawa M, Houdou S, Mito T et al (1992) Development of myelination in the human fetal and infant cerebrum: a myelin basic protein immunohistochemical study. Brain Dev 14:1–6
Garel C, Chantrel E, Elmaleh M et al (2003) Fetal MRI: normal gestational landmarks for cerebral biometry, gyration and myelination. Childs Nerv Syst 19:422–425
Cassart M, Garel C (2020) European overview of current practice of fetal imaging by pediatric radiologists: a new task force is launched. Pediatr Radiol 50:1794–1798
Brunet O, Lézine I, Josse D (1997) Brunet-Lézine révisé: échelle de développement psychomoteur de la première enfance : manuel BLR-C. Issy-Les-Moulineaux (France): Etablissements d’Applications Psychotechniques
Acknowledgements
We thank Dr. Catherine Garel for her thorough review and her thoughtful remarks during the writing of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Letissier, C., Crombé, A., Chérier, L. et al. Brain fetal magnetic resonance imaging to evaluate maturation of normal white matter during the third trimester of pregnancy. Pediatr Radiol 51, 1826–1838 (2021). https://doi.org/10.1007/s00247-021-05064-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05064-1