Abstract
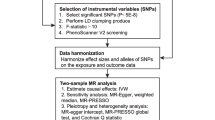
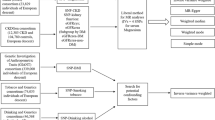
The causal links between urinary uromodulin (uUMOD) and kidney stone disease (KSD) are still not clarified in general population. We assessed their relationships combining 2-sample Mendelian randomization (MR) and multivariable (MVMR) designs among general population of European ancestry. The summary information for uUMOD indexed to creatinine levels (29,315 individuals) and KSD (395,044 individuals) were from 2 independent genome-wide association studies (GWAS). The primary causal effects of exposures on outcomes were evaluated using inverse variance-weighted (IVW) regression model. Multiple sensitivity analyses were also performed. In 2-sample MR, we found that 1-unit higher genetically predicted uUMOD levels were associated with a lower risk of KSD (OR = 0.62; 95% CI 0.55–0.71; P = 2.83E−13). In reverse, we did not find the effect of KSD on uUOMD using IVW (beta = 0.00; 95% CI − 0.06–0.05; P = 0.872) and other sensitivity analyses. In MVMR, uUMOD indexed to creatinine levels were directly associated with the risk of KSD after introducing eGFR, SBP, urinary sodium or all three factors (OR = 0.71; 95% CI 0.64–0.79; P = 1.57E−09). Furthermore, our study supported that the protective effect of uUMOD on KSD may be partially mediated by eGFR (beta = − 0.09; 95% CI − 0.13 to − 0.06; mediation proportion = 20%). Our study supported that the protective effect of genetically predicted higher uUMOD levels on KSD may be partially mediated by eGFR decline, but not via SBP or urinary sodium. uUMOD might be a treatment target in preventing KSD in general population.


Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary information files.
References
Schaeffer C, Devuyst O, Rampoldi L (2021) Uromodulin: roles in health and disease. Annu Rev Physiol 83:477–501
Glauser A, Hochreiter W, Jaeger P, Hess B (2000) Determinants of urinary excretion of Tamm-Horsfall protein in non-selected kidney stone formers and healthy subjects. Nephrol Dial Transplant 15:1580–1587
Lau WH, Leong WS, Ismail Z, Gam LH (2008) Qualification and application of an ELISA for the determination of Tamm Horsfall protein (THP) in human urine and its use for screening of kidney stone disease. Int J Biol Sci 4:215–222
Chaiyarit S, Thongboonkerd V (2022) Oxidized forms of uromodulin promote calcium oxalate crystallization and growth, but not aggregation. Int J Biol Macromol 214:542–553
Noonin C, Peerapen P, Yoodee S, Kapincharanon C, Kanlaya R, Thongboonkerd V (2022) Systematic analysis of modulating activities of native human urinary Tamm−Horsfall protein on calcium oxalate crystallization, growth, aggregation, crystal-cell adhesion and invasion through extracellular matrix. Chem Biol Interact 357:109879
Ponte B, Sadler MC, Olinger E, Vollenweider P, Bochud M, Padmanabhan S et al (2021) Mendelian randomization to assess causality between uromodulin, blood pressure and chronic kidney disease. Kidney Int 100:1282–1291
Uribarri J (2020) Chronic kidney disease and kidney stones. Curr Opin Nephrol Hypertens 29:237–242
Sekula P, Del Greco MF, Pattaro C, Kottgen A (2016) Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 27:3253–3265
Lin BB, Huang RH, Lin BL, Hong YK, Lin ME, He XJ (2020) Associations between nephrolithiasis and diabetes mellitus, hypertension and gallstones: a meta-analysis of cohort studies. Nephrology (Carlton) 25:691–699
Afsar B, Kiremit MC, Sag AA, Tarim K, Acar O, Esen T et al (2016) The role of sodium intake in nephrolithiasis: epidemiology, pathogenesis, and future directions. Eur J Intern Med 35:16–19
Sanderson E, Davey Smith G, Windmeijer F, Bowden J (2019) An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol 48:713–727
Sanderson E (2021) Multivariable Mendelian Randomization and Mediation. Cold Spring Harb Perspect Med 11(2):a038984. https://doi.org/10.1101/cshperspect.a038984
Joseph CB, Mariniello M, Yoshifuji A, Schiano G, Lake J, Marten J et al (2022) Meta-GWAS reveals novel genetic variants associated with urinary excretion of uromodulin. J Am Soc Nephrol 33:511–529
Howles SA, Wiberg A, Goldsworthy M, Bayliss AL, Gluck AK, Ng M et al (2019) Genetic variants of calcium and vitamin D metabolism in kidney stone disease. Nat Commun 10:5175
Wuttke M, Li Y, Li M, Sieber KB, Feitosa MF, Gorski M et al (2019) A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 51:957–972
Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H et al (2018) Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet 50:1412–1425
Pazoki R, Evangelou E, Mosen-Ansorena D, Pinto RC, Karaman I, Blakeley P et al (2019) GWAS for urinary sodium and potassium excretion highlights pathways shared with cardiovascular traits. Nat Commun 10:3653
Elsworth B, Lyon M, Alexander T, Liu Y, Matthews P, Hallett J et al (2020) The MRC IEU OpenGWAS data infrastructure. Biorxiv. https://doi.org/10.1101/2020.08.10.244293
Verbanck M, Chen CY, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50:693–698
Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37:658–665
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40:304–314
Hartwig FP, Davey Smith G, Bowden J (2017) Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol 46:1985–1998
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–525
Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA et al (2019) Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol 48:728–742
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J (2017) A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med 36:1783–1802
Verbanck M, Chen C-Y, Neale B et al (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50(5):693–698
Burgess S, Thompson DJ, Rees JMB, Day FR, Perry JR, Ong KK (2017) Dissecting causal pathways using Mendelian randomization with summarized genetic data: application to age at menarche and risk of breast cancer. Genetics 207:481–487
Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J et al (2021) Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol 36:465–478
Davies NM, Holmes MV, Davey SG (2018) Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362:k601
Burgess S, Thompson SG, Collaboration CCG (2011) Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 40:755–764
Gorski M, van der Most PJ, Teumer A, Chu AY, Li M, Mijatovic V et al (2017) 1000 genomes-based meta-analysis identifies 10 novel loci for kidney function. Sci Rep 7:45040
Yu Z, Coresh J, Qi G, Grams M, Boerwinkle E, Snieder H et al (2020) A bidirectional Mendelian randomization study supports causal effects of kidney function on blood pressure. Kidney Int 98:708–716
Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR (2004) Tamm−Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int 66:1159–1166
Liu J, Tio MC, Verma A, Schmidt IM, Ilori TO, Knauf F et al (2022) Determinants and Outcomes associated with urinary calcium excretion in chronic kidney disease. J Clin Endocrinol Metab 107:e281–e292
Liu Y, Mo L, Goldfarb DS, Evan AP, Liang F, Khan SR et al (2010) Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm−Horsfall protein. Am J Physiol Renal Physiol 299:F469–F478
Wolf MT, Wu XR, Huang CL (2013) Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int 84:130–137
Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J et al (2011) Activation of the bumetanide-sensitive Na+, K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm−Horsfall protein in a chloride-sensitive manner. J Biol Chem 286:30200–30210
Torffvit O, Melander O, Hulten UL (2004) Urinary excretion rate of Tamm−Horsfall protein is related to salt intake in humans. Nephron Physiol 97:p31–p36
Liu Y, Goldfarb DS, El-Achkar TM, Lieske JC, Wu XR (2018) Tamm−Horsfall protein/uromodulin deficiency elicits tubular compensatory responses leading to hypertension and hyperuricemia. Am J Physiol Renal Physiol 314:F1062–F1076
Funding
This study was supported by the Foundation of Science & Technology Department of Sichuan Province [2021YFS0116, 2022YFS0304] and the Post-Doctor Research Project, West China Hospital, Sichuan University, Grant/Award Number: 2020HXBH016.
Author information
Authors and Affiliations
Contributions
Conceptualization and original draft preparation: ZJ and CY. Data collection: ZX. Data analysis: HL. Resources and supervision: XJ and KW. All authors read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval
No ethics committee approval was conducted since this study didn’t involve animal and human experimentations or data containing individual information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jian, Z., Yuan, C., Xiong, Z. et al. Kidney function may partially mediated the protective effect of urinary uromodulin on kidney stone. Urolithiasis 51, 65 (2023). https://doi.org/10.1007/s00240-023-01441-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00240-023-01441-7