Abstract
Background
Dabrafenib and trametinib represent targeted therapy options under investigation for treatment of gliomas harboring BRAF V600 mutations. We systematically reviewed the literature and conducted meta-analyses to assess the efficacy and safety of these agents.
Methods
PubMed, Embase, and Scopus were searched from inception to September 2023 for studies examining dabrafenib and/or trametinib for gliomas. Outcomes included response rates (ORR, CR, PR), progression rates (PD), 6- and 12-month PFS, adverse events, and dosing modifications. Meta-analyses were conducted using random effect models.
Results
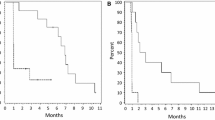
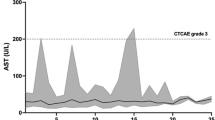
Nine studies met the inclusion criteria. Meta-analysis demonstrated overall response rates (ORR) of 50% (95% confidence interval (CI): 35–65%) for low-grade gliomas (LGG) and 40% (95% CI: 29–51%) for high-grade gliomas (HGG). Pooled ORR was 45% (95% CI: 36–54%) for both glioma grades. The complete response rate was 13% (95% CI: 05–27%) for HGG and 5% (95% CI: 1–10%) for both LGG and HGG. Six-month progression-free survival (PFS) rates reached 87% in LGG and 67% in HGG and a pooled 6-month PFS 78% (95% CI: 58–98%), declining at 12 months to 67% and 44%, respectively, with a pooled 12-month PFS 56% (95% CI: 34–79%). Grade 1–4 adverse events occurred in 100% of LGG and 63% of HGG patients.
Conclusions
Dabrafenib and trametinib demonstrate promising anti-tumor efficacy in gliomas, particularly low-grade tumors, achieving durable disease stabilization in many patients. However, toxicity significantly limited tolerability. Additional research should further examine efficacy and refine safe administration protocols across glioma subtypes.












Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are available from the corresponding author, M.A Habibi, upon reasonable request.
References
Bouffet E et al (2023) Efficacy and safety of trametinib monotherapy or in combination with dabrafenib in pediatric BRAF V600-mutant low-grade glioma. J Clin Oncol 41(3):664–674
Hargrave DR, Investigators involved in the high-grade glioma cohort et al (2023) Phase II trial of dabrafenib plus trametinib in relapsed/refractory BRAF V600-mutant pediatric high-grade glioma. J Clin Oncol 41(33):5174–5183. https://doi.org/10.1200/JCO.23.00558. Epub 2023 Aug 29
Wen PY et al (2022) Dabrafenib plus trametinib in patients with BRAF(V600E)-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol 23(1):53–64
Johanns TM et al (2018) Rapid clinical and radiographic response with combined dabrafenib and trametinib in adults with BRAF-mutated high-grade glioma. J Natl Compr Canc Netw 16(1):4–10
Geoerger B et al (2020) Dabrafenib + trametinib combination therapy in pediatric patients with BRAF V600-mutant low-grade glioma: safety and efficacy results. J Clin Oncol 38(15):10506–10506
Berzero G et al (2021) Sustained tumor control with MAPK inhibition in BRAF V600-mutant adult glial and glioneuronal tumors. Neurology 97(7):e673–e683
Subbiah V et al (2023) Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med 29(5):1103–1112
Matthew JP et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Deeks JJ, Higgins JP, Altman DG, Cochrane Statistical Methods Group (2019) Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions. https://doi.org/10.1002/9781119536604.ch10
Bouffet E et al (2023) Dabrafenib plus trametinib in pediatric glioma with BRAF V600 mutations. N Engl J Med 389(12):1108–1120
Leclair NK et al (2022) Early experience with targeted therapy as a first-line adjuvant treatment for pediatric low-grade glioma. Neurosurg Focus 53(6):E15
Habibi MA, Fakhfouri A, Mirjani MS, Razavi A, Mortezaei A, Soleimani Y, Lotfi S, Arabi S, Heidaresfahani L, Sadeghi S, Minaee P, Eazi S, Rashidi F, Shafizadeh M, Majidi S (2024) Prediction of cerebral aneurysm rupture risk by machine learning algorithms: a systematic review and meta-analysis of 18,670 participants. Neurosurg Rev 47(1):34. https://doi.org/10.1007/s10143-023-02271-2. PMID: 38183490
Funding
There is no funding source with authors to declare.
Author information
Authors and Affiliations
Contributions
MA. H and MS.M contributed to the study conception and design, and edited the manuscript. MA.H and P.D and MH.A analyzed the data and wrote the first draft of the manuscript. O.A collected data. MA.H made a critical revision of the manuscript. All authors commented on previous versions of the manuscript and revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study is deemed to exempt to receive ethical approval.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habibi, M.A., Mirjani, M.S., Ahmadvand, M.H. et al. The safety and efficacy of dabrafenib and trametinib in patients with glioma: A systematic review and meta-analysis. Eur J Clin Pharmacol 80, 639–656 (2024). https://doi.org/10.1007/s00228-024-03635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-024-03635-3