Abstract
Purpose
There are limited data regarding the safety of direct oral anticoagulants (DOACs) during breastfeeding. The aim of the present study is to investigate the extent of excretion of DOACs into human milk according to the available clinical and experimental studies.
Methods
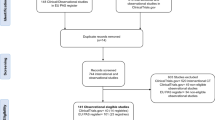
On 16th January 2021, we systematically searched PubMed, Scopus, Embase, and Web of Science for all studies which investigated DOACs in breastfeeding without any time frame and language limitation. Search keywords were [breastfeeding, breast feeding, breastfed, lactation, milk secretion OR milk] AND [apixaban OR Eliquist OR rivaroxaban OR Xarelto OR edoxaban OR Savaysa OR dabigatran OR Pradaxa OR dabigatran etexilate OR dabigatran etexilate mesylate OR direct oral anticoagulant OR DOAC OR new oral anticoagulant OR NOAC]. Finally, we identified six articles which reported DOAC use during breastfeeding or lactation.
Results and conclusion
According to the available limited data, dabigatran has the least excretion in human breast milk. Rivaroxaban and dabigatran both have acceptable milk excretion cutoffs, whereas apixaban milk excretion is greater than the maximum allowed range. Further well-designed studies with larger sample sizes are required to generate consistent comparable data and clarify benefits and risks of each DOAC during breastfeeding.

Similar content being viewed by others
Availability of data and material
Data analyzed in this study were a reanalysis of existing data, which are openly available at locations cited in the reference section.
References
Chan N, Sobieraj-Teague M, Eikelboom JW (2020) Direct oral anticoagulants: evidence and unresolved issues. Lancet 396(10264):1767–1776. https://doi.org/10.1016/s0140-6736(20)32439-9
Franco Moreno AI, Martín Díaz RM, García Navarro MJ (2018) Direct oral anticoagulants: an update. Med Clin (Barc) 151(5):198–206. https://doi.org/10.1016/j.medcli.2017.11.042
Chen A, Stecker E, Warden BA (2020) Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc 9(13):e017559. https://doi.org/10.1161/jaha.120.017559
Food and Drug Administration. Pregnant women: scientific and ethical considerations for inclusion in clinical trials. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pregnant-women-scientific-and-ethical-considerations-inclusion-clinical-trials. Updated 2018. Accecced 18 January 2021
Anderson PO, Sauberan JB (2016) Modeling drug passage into human milk. Clin Pharmacol Ther 100(1):42–52. https://doi.org/10.1002/cpt.377
Food and Drug Administration. Clinical lactation studies: considerations for study design. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-lactation-studies-considerations-study-design. Updated 2019. Accecced 18 January 2021
Ballard O, Morrow AL (2013) Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 60(1):49–74. https://doi.org/10.1016/j.pcl.2012.10.002
Begg EJ, Duffull SB, Hackett LP, Ilett KF (2002) Studying drugs in human milk: time to unify the approach. J Hum Lact 18(4):323–332. https://doi.org/10.1177/089033402237904
Ito S, Lee A (2003) Drug excretion into breast milk–overview. Adv Drug Deliv Rev 55(5):617–627. https://doi.org/10.1016/s0169-409x(03)00034-6
Zhao Y, Arya R, Couchman L, Patel JP (2020) Are apixaban and rivaroxaban distributed into human breast milk to clinically relevant concentrations? Blood 136(15):1783–1785. https://doi.org/10.1182/blood.2020006231
Wang L, He K, Maxwell B, Grossman SJ, Tremaine LM, Humphreys WG, Zhang D (2011) Tissue distribution and elimination of [14C]apixaban in rats. Drug Metab Dispos 39(2):256–264. https://doi.org/10.1124/dmd.110.036442
Wiesen MHJ, Blaich C, Müller C, Streichert T, Pfister R, Michels G (2016) The direct factor Xa inhibitor rivaroxaban passes into human breast milk. Chest 150(1):e1–e4. https://doi.org/10.1016/j.chest.2016.01.021
Saito J, Kaneko K, Yakuwa N, Kawasaki H, Yamatani A, Murashima A (2019) Rivaroxaban concentration in breast milk during breastfeeding: a case study. Breastfeed Med 14(10):748–751. https://doi.org/10.1089/bfm.2019.0230
Muysson M, Marshall K, Datta P, Rewers-Felkins K, Baker T, Hale TW (2020) Rivaroxaban treatment in two breastfeeding mothers: a case series. Breastfeed Med 15(1):41–43. https://doi.org/10.1089/bfm.2019.0124
Ayuk P, Kampouraki E, Truemann A, Sidgwick F, McDonald L, Bingham J, Murphy P, Kamali F (2020) Investigation of dabigatran secretion into breast milk: implications for oral thromboprophylaxis in post-partum women. Am J Hematol 95(1):E10–E13. https://doi.org/10.1002/ajh.25652
Ito S (2000) Drug therapy for breast-feeding women. N Engl J Med 343(2):118–126. https://doi.org/10.1056/NEJM200007133430208
Remko M (2009) Molecular structure, lipophilicity, solubility, absorption, and polar surface area of novel anticoagulant agents. J Mol Struct (Thoechem) 916(1–3):76–85. https://doi.org/10.1016/j.theochem.2009.09.011
Kenet G (2021) Dabigatran etexilate and treatment of acute venous thromboembolism in children. Lancet Haematol 8(1):e2–e3. https://doi.org/10.1016/s2352-3026(20)30397-5
Brandão LR, Albisetti M, Halton J, Bomgaars L, Chalmers E, Mitchell LG, Nurmeev I, Svirin P, Kuhn T, Zapletal O, Tartakovsky I, Simetzberger M, Huang F, Sun Z, Kreuzer J, Gropper S, Brueckmann M, Luciani M (2020) Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children. Blood 135(7):491–504. https://doi.org/10.1182/blood.2019000998
Male C, Lensing AWA, Palumbo JS, Kumar R, Nurmeev I, Hege K, Bonnet D, Connor P, Hooimeijer HL, Torres M, Chan AKC, Kenet G, Holzhauer S, Santamaría A, Amedro P, Chalmers E, Simioni P, Bhat RV, Yee DL, Lvova O, Beyer-Westendorf J, Biss TT, Martinelli I, Saracco P, Peters M, Kállay K, Gauger CA, Massicotte MP, Young G, Pap AF, Majumder M, Smith WT, Heubach JF, Berkowitz SD, Thelen K, Kubitza D, Crowther M, Prins MH, Monagle P (2020) Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol 7(1):e18–e27. https://doi.org/10.1016/s2352-3026(19)30219-4
Young G, Lensing AWA, Monagle P, Male C, Thelen K, Willmann S, Palumbo JS, Kumar R, Nurmeev I, Hege K, Bajolle F, Connor P, Hooimeijer HL, Torres M, Chan AKC, Kenet G, Holzhauer S, Santamaría A, Amedro P, Beyer-Westendorf J, Martinelli I, Massicotte MP, Smith WT, Berkowitz SD, Schmidt S, Price V, Prins MH, Kubitza D (2020) Rivaroxaban for treatment of pediatric venous thromboembolism. An Einstein-Jr phase 3 dose-exposure-response evaluation. J Thromb Haemost 18 (7):1672–1685. https://doi.org/10.1111/jth.14813
Thom K, Lensing AWA, Nurmeev I, Bajolle F, Bonnet D, Kenet G, Massicotte MP, Karakas Z, Palumbo JS, Saracco P, Amedro P, Chain J, Chan AK, Ikeyama T, Lam JCM, Gauger C, Pap ÁF, Majumder M, Kubitza D, Smith WT, Berkowitz SD, Prins MH, Monagle P, Young G, Male C (2020) Safety and efficacy of anticoagulant therapy in pediatric catheter-related venous thrombosis (EINSTEIN-Jr CVC-VTE). Blood Adv 4(19):4632–4639. https://doi.org/10.1182/bloodadvances.2020002637
Gong IY, Kim RB (2013) Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol 29(7):S24-33. https://doi.org/10.1016/j.cjca.2013.04.002
Dubois V, Dincq A-S, Douxfils J, Ickx B, Samama C-M, Dogné J-M, Gourdin M, Chatelain B, Mullier F, Lessire S (2017) Perioperative management of patients on direct oral anticoagulants. Thromb J 15:14. https://doi.org/10.1186/s12959-017-0137-1
Hodin S, Basset T, Jacqueroux E, Delezay O, Clotagatide A, Perek N, Mismetti P, Delavenne X (2018) In vitro comparison of the role of P-glycoprotein and breast cancer resistance protein on direct oral anticoagulants disposition. Eur J Drug Metab Pharmacokinet 43(2):183–191. https://doi.org/10.1007/s13318-017-0434-x
Ahmadzai H, Tee L, Crowe A (2014) Pharmacological role of efflux transporters: clinical implications for medication use during breastfeeding. World J Pharmacol 3(4):153–161. https://doi.org/10.5497/wjp.v3.i4.153
Payne RM, Burns KM, Glatz AC, Li D, Li X, Monagle P, Newburger JW, Swan EA, Wheaton O, Male C (2019) A multi-national trial of a direct oral anticoagulant in children with cardiac disease: design and rationale of the safety of apixaban on pediatric heart disease on the prevention of embolism (saxophone) study. Am Heart J 217:52–63. https://doi.org/10.1016/j.ahj.2019.08.002
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The literature search and data analysis were performed by Maryam Daei and Zinat Heidari. The first draft of the manuscript was written by Maryam Daei and Zinat Heidari and Hossein Khalili revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Daei, M., Khalili, H. & Heidari, Z. Direct oral anticoagulant safety during breastfeeding: a narrative review. Eur J Clin Pharmacol 77, 1465–1471 (2021). https://doi.org/10.1007/s00228-021-03154-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03154-5