Abstract
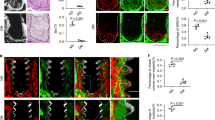
Together with diabetic osteoporosis (DOP), diabetes patients experience poor peri-implant osteogenesis following implantation for dentition defects. Zoledronate (ZOL) is widely used to treat osteoporosis clinically. To evaluate the mechanism of ZOL for the treatment of DOP, experiments with DOP rats and high glucose-grown MC3T3-E1 cells were used. The DOP rats treated with ZOL and/or ZOL implants underwent a 4-week implant-healing interval, and then microcomputed tomography, biomechanical testing, and immunohistochemical staining were performed to elucidate the mechanism. In addition, MC3T3-E1 cells were maintained in an osteogenic medium with or without ZOL to confirm the mechanism. The cell migration, cellular actin content, and osteogenic differentiation were evaluated by a cell activity assay, a cell migration assay, as well as alkaline phosphatase, alizarin red S, and immunofluorescence staining. The mRNA and protein expression of adenosine monophosphate-activated protein kinase (AMPK), phosphorylated AMPK (p-AMPK), osteoprotegerin (OPG), receptor activator of nuclear factor kappa B ligand (RANKL), bone morphogenetic protein 2 (BMP2), and collagen type I (Col-I) were detected using real-time quantitative PCRs and western blot assays, respectively. In the DOP rats, ZOL markedly improved osteogenesis, enhanced bone strength and increased the expression of AMPK, p-AMPK, and Col-I in peri-implant bones. The in vitro findings showed that ZOL reversed the high glucose-induced inhibition of osteogenesis via the AMPK signaling pathway. In conclusion, the ability of ZOL to promote osteogenesis in DOP by targeting AMPK signaling suggests that therapy with ZOL, particularly simultaneous local and systemic administration, may be a unique approach for future implant repair in diabetes patients.







Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Kotsovilis S, Karoussis IK, Fourmousis I (2006) A comprehensive and critical review of dental implant placement in diabetic animals and patients. Clin Oral Implants Res 17:587–599
Moy PK, Medina D, Shetty V, Aghaloo TL (2005) Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants 20:569–577
Dalle Carbonare L, Mottes M, Malerba G, Mori A, Zaninotto M, Plebani M, Dellantonio A, Valenti MT (2017) Enhanced osteogenic differentiation in Zoledronate-treated osteoporotic patients. Int J Mol Sci 18:1261
Barale M, Sigrist S, Bioletto F, Maiorino F, Ghigo E, Mazzetti R, Procopio M (2021) Long-term efficacy of intensive zoledronate therapy and predictors of retreatment in paget’s disease of bone. Calcif Tissue Int 109:383–392
Bonnet N, Lesclous P, Saffar JL, Ferrari S (2013) Zoledronate effects on systemic and jaw osteopenias in ovariectomized periostin-deficient mice. PLoS ONE 8:e58726
Cui M, Yu LZ, Zhang N, Wang LJ, Sun J, Cong J (2016) Zoledronic acid improves bone quality in the Streptozotocin-induced diabetes rat through affecting the expression of the osteoblast-regulating transcription factors. Exp Clin Endocrinol Diabetes 127:68–75
Huang KC, Huang TW, Chuang PY, Yang TY, Chang SF (2019) Zoledronate induces cell cycle arrest and differentiation by upregulating p21 in mouse MC3T3-E1 preosteoblasts. Int J Med Sci 16:751–756
Li Y, Su J, Sun W, Cai L, Deng Z (2018) AMP-activated protein kinase stimulates osteoblast differentiation and mineralization through autophagy induction. Int J Mol Med 41:2535–2544
Jeyabalan J, Shah M, Viollet B, Chenu C (2012) AMP-activated protein kinase pathway and bone metabolism. J Endocrinol 212:277–290
Kasai T, Bandow K, Suzuki H, Chiba N, Kakimoto K, Ohnishi T, Kawamoto S, Nagaoka E, Matsuguchi T (2009) Osteoblast differentiation is functionally associated with decreased AMP kinase activity. J Cell Physiol 221:740–749
Oh SJ, Gu DR, Jin SH, Park KH, Lee SH (2016) Cytosolic malate dehydrogenase regulates RANKL-mediated osteoclastogenesis via AMPK/c-Fos/NFATc1 signaling. Biochem Biophys Res Commun 475:125–132
Wang Y-g, Qu X-h, Yang Y, Han X-g, Wang L, Qiao H, Fan Q-m, Tang T-t, Dai K-r (2016) AMPK promotes osteogenesis and inhibits adipogenesis through AMPK-Gfi1-OPN axis. Cell Signal 28:1270–1282
Qi M, Hu J, Li J, Li J, Dong W, Feng X, Yu J (2012) Effect of zoledronate acid treatment on osseointegration and fixation of implants in autologous iliac bone grafts in ovariectomized rabbits. Bone 50:119–127
Yang LC, Lin SW, Li IC, Chen YP, Tzu SY, Chou W, Chen CC, Lin WC, Chen YL, Lin WH (2020) Lactobacillus plantarum GKM3 and Lactobacillus paracasei GKS6 Supplementation Ameliorates bone loss in Ovariectomized mice by promoting Osteoblast differentiation and inhibiting Osteoclast formation. Nutrients 12:1914
Castellani C, Lindtner RA, Hausbrandt P, Tschegg E, Stanzl-Tschegg SE, Zanoni G, Beck S, Weinberg AM (2011) Bone-implant interface strength and osseointegration: Biodegradable magnesium alloy versus standard titanium control. Acta Biomater 7:432–440
Sordi MB, Curtarelli RB, da Silva IT, Fongaro G, Benfatti CAM, de Souza MR, Cabral da Cruz AC (2021) Effect of dexamethasone as osteogenic supplementation in in vitro osteogenic differentiation of stem cells from human exfoliated deciduous teeth. J Mater Sci Mater Med 32:1
Tatarakis N, Kinney JS, Inglehart M, Braun TM, Shelburne C, Lang NP, Giannobile WV, Oh TJ (2014) Clinical, microbiological, and salivary biomarker profiles of dental implant patients with type 2 diabetes. Clin Oral Implants Res 25:803–812
Zou GK, Song YL, Zhou W, Yu M, Liang LH, Sun DC, Li DH, Deng ZX, Zhu WZ (2012) Effects of local delivery of bFGF from PLGA microspheres on osseointegration around implants in diabetic rats. Oral Surg Oral Med Oral Pathol Oral Radiol 114:284–289
Shibamoto A, Ogawa T, Duyck J, Vandamme K, Naert I, Sasaki K (2018) Effect of high-frequency loading and parathyroid hormone administration on peri-implant bone healing and osseointegration. Int J Oral Sci 10:6
Liu H, Zhang N, Liu Y, Liu L, Yin G, En L (2018) Effect of Human Wnt10b Transgene Overexpression on Peri-Implant Osteogenesis in Ovariectomized Rats. Hum Gene Ther 29:1416–1427
Hua Y, Bi R, Li Z, Li Y (2020) Resveratrol treatment promotes titanium implant osseointegration in diabetes mellitus rats. J Orthop Res 38:2113–2119
Zhou R, Ma Y, Qiu S, Gong Z, Zhou X (2020) Metformin promotes cell proliferation and osteogenesis under high glucose condition by regulating the ROS-AKT-mTOR axis. Mol Med Rep 22:3387–3395
Yang J, Ma C, Zhang M (2019) High glucose inhibits osteogenic differentiation and proliferation of MC3T3-E1 cells by regulating P2X7. Mol Med Rep 20:5084–5090
Yang XJ, Wang FQ, Lu CB, Zou JW, Hu JB, Yang Z, Sang HX, Zhang Y (2020) Modulation of bone formation and resorption using a novel zoledronic acid loaded gelatin nanoparticles integrated porous titanium scaffold: an in vitro and in vivo study. Biomed Mater 15:055013
Gao Y, Zou S, Liu X, Bao C, Hu J (2009) The effect of surface immobilized bisphosphonates on the fixation of hydroxyapatite-coated titanium implants in ovariectomized rats. Biomaterials 30:1790–1796
von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, Rubash HE, Shanbhag AS (2005) Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials 26:6941–6949
Basudan AM, Shaheen MY, Niazy AA, van den Beucken J, Jansen JA, Alghamdi HS (2020) Histomorphometric evaluation of peri-implant bone response to intravenous administration of Zoledronate (Zometa(®)) in an Osteoporotic rat model. Materials (Basel) 13:5248
Pyo SW, Kim YM, Kim CS, Lee IS, Park JU (2014) Bone formation on biomimetic calcium phosphate-coated and zoledronate-immobilized titanium implants in osteoporotic rat tibiae. Int J Oral Maxillofac Implants 29:478–484
Kajiwara H, Yamaza T, Yoshinari M, Goto T, Iyama S, Atsuta I, Kido MA, Tanaka T (2005) The bisphosphonate pamidronate on the surface of titanium stimulates bone formation around tibial implants in rats. Biomaterials 26:581–587
Gao Y, Luo E, Hu J, Xue J, Zhu S, Li J (2009) Effect of combined local treatment with zoledronic acid and basic fibroblast growth factor on implant fixation in ovariectomized rats. Bone 44:225–232
Manfredi M, Merigo E, Guidotti R, Meleti M, Vescovi P (2011) Bisphosphonate-related osteonecrosis of the jaws: a case series of 25 patients affected by osteoporosis. Int J Oral Maxillofac Surg 40:277–284
Dodson TB (2009) Intravenous bisphosphonate therapy and bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 67:44–52
Ikeda H, Yoshiga D, Kokabu S, Ariyoshi W, Tsurushima H, Sakaguchi O, Tanaka J, Kaneko J, Habu M, Sasaguri M, Jimi E, Nishihara T, Yoshioka I, Tominaga K (2019) Evaluation of therapeutic effects of teriparatide in a rat model of zoledronic acid-induced bisphosphonate-related osteonecrosis. J Oral and Maxillofacial Surg, Med, Pathol 31:333–341
Tsurushima H, Kokuryo S, Sakaguchi O, Tanaka J, Tominaga K (2013) Bacterial promotion of bisphosphonate-induced osteonecrosis in Wistar rats. Int J Oral Maxillofac Surg 42:1481–1487
Gu Y, Hou T, Qin Y, Dong W (2022) Zoledronate promotes osteoblast differentiation in high-glucose conditions via the p38MAPK pathway. Cell Biol Int. https://doi.org/10.1002/cbin.11921
Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D, Trajkovic V (2013) Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone 52:524–531
Zhang X, Huang F, Chen X, Wu X, Zhu J (2020) Ginsenoside Rg3 attenuates ovariectomy-induced osteoporosis via AMPK/mTOR signaling pathway. Drug Dev Res 81:875–884
Chen M, Jing D, Ye R, Yi J, Zhao Z (2021) PPARβ/δ accelerates bone regeneration in diabetic mellitus by enhancing AMPK/mTOR pathway-mediated autophagy. Stem Cell Res Ther 12:566
Wang N, Wang L, Yang J, Wang Z, Cheng L (2021) Quercetin promotes osteogenic differentiation and antioxidant responses of mouse bone mesenchymal stem cells through activation of the AMPK/SIRT1 signaling pathway. Phytother Res 35:2639
Xiao J, Li W, Li G, Tan J, Dong N (2022) STK11 overexpression prevents glucocorticoid-induced osteoporosis via activating the AMPK/SIRT1/PGC1α axis. Hum Cell 35:1045–1059
Ma L, Gong F, Xu J, Yang J (2021) Uncarboxylated osteocalcin reverses the high glucose-induced inhibition of the osteogenic differentiation of MC3T3E1 cells via the GPRC6A/cAMP/PKA/AMPK signaling pathway. Int J Mol Med. https://doi.org/10.3892/ijmm.2021.4924
Baron SJ, Li J, Russell RR 3rd, Neumann D, Miller EJ, Tuerk R, Wallimann T, Hurley RL, Witters LA, Young LH (2005) Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ Res 96:337–345
Wu L, Liu C, Chang DY, Zhan R, Zhao M, Man Lam S, Shui G, Zhao MH, Zheng L, Chen M (2021) The attenuation of diabetic nephropathy by Annexin A1 via regulation of lipid metabolism through the AMPK/PPARα/CPT1b pathway. Diabetes 70:2192–2203
Jang WG, Kim EJ, Lee KN, Son HJ, Koh JT (2011) AMP-activated protein kinase (AMPK) positively regulates osteoblast differentiation via induction of Dlx5-dependent Runx2 expression in MC3T3E1 cells. Biochem Biophys Res Commun 404:1004–1009
Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T (2009) Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. Am J Physiol Endocrinol Metab 296:E139-146
Wang CK, Ho ML, Wang GJ, Chang JK, Chen CH, Fu YC, Fu HH (2009) Controlled-release of rhBMP-2 carriers in the regeneration of osteonecrotic bone. Biomaterials 30:4178–4186
Fan YS, Li Q, Hamdan N, Bian YF, Zhuang S, Fan K, Liu ZJ (2018) Tetrahydroxystilbene glucoside regulates proliferation, differentiation, and OPG/RANKL/M-CSF expression in MC3T3-E1 cells via the PI3K/Akt pathway. Molecules 23:2306
Atkins GJ, Kostakis P, Pan B, Farrugia A, Gronthos S, Evdokiou A, Harrison K, Findlay DM, Zannettino AC (2003) RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res 18:1088–1098
Tong X, Gu J, Song R, Wang D, Sun Z, Sui C, Zhang C, Liu X, Bian J, Liu Z (2018) Osteoprotegerin inhibit osteoclast differentiation and bone resorption by enhancing autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J Cell Biochem. https://doi.org/10.1002/jcb.27468
Maria S, Samsonraj RM, Munmun F, Glas J, Silvestros M, Kotlarczyk MP, Rylands R, Dudakovic A, van Wijnen AJ, Enderby LT, Lassila H, Dodda B, Davis VL, Balk J, Burow M, Bunnell BA, Witt-Enderby PA (2018) Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J Pineal Res 64:e12465
Dong W, Qi M, Wang Y, Feng X, Liu H (2018) Zoledronate and high glucose levels influence osteoclast differentiation and bone absorption via the AMPK pathway. Biochem Biophys Res Commun 505:1195–1202
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81600844); Science and Technology Project of Hebei Education Department (Grant No. QN2020438); Natural Science Foundation of Hebei Province (Grant No. H2017209114).
Author information
Authors and Affiliations
Contributions
YZ: conceptualization, methodology, investigation, data curation, writing—original draft. SJ: conceptualization, methodology, investigation, data curation, writing—original draft. GW: methodology, data curation, software, visualization. SX: methodology, data curation. ZS: methodology. MQ: data curation. YL: methodology. WB: resources, funding acquisition. WD: conceptualization, writing—review & editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Disclosure
Wei Dong received grants from the Science and Technology Project of Hebei Education Department (Grant No. QN2020438) and the Natural Science Foundation of Hebei Province (Grant No. H2017209114). Wenjuan Bi received grants from the National Natural Science Foundation of China (Grant No. 81600844). Yan Zhang, Shunyi Jia, Guochen Wen, Shanen Xie, Zhiqiang Song, Mengchun Qi and Yongqiang Liang declare that they have no conflict of interest to report.
Ethical Approval
The study was approved by the Animal Experimental Ethical Committee of North China University of Science and Technology (No. LX2021106) and performed in accordance with the regulations and codes of practice for laboratory animal management and ethical requirements.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Jia, S., Wen, G. et al. Zoledronate Promotes Peri-Implant Osteogenesis in Diabetic Osteoporosis by the AMPK Pathway. Calcif Tissue Int 113, 329–343 (2023). https://doi.org/10.1007/s00223-023-01112-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01112-0