Abstract
Summary
Denosumab discontinuation results in accelerated bone remodeling, decreased bone mineral density (BMD), and an increased risk of multiple vertebral fractures. Bisphosphonates are at least partially effective at inhibiting these consequences but there have been no randomized clinical trials assessing the efficacy of alternative antiresorptives.
Purpose
The aim of this study was to evaluate the comparative efficacy of alendronate and the SERM, raloxifene, in preventing the post-denosumab high-turnover bone loss.
Methods
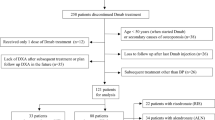
We conducted an open-label randomized controlled trial in which 51 postmenopausal women at increased risk of fracture were randomized with equal probability to receive 12-months of denosumab 60-mg 6-monthly followed by 12-months of either alendronate 70-mg weekly or raloxifene 60-mg daily. Serum bone remodeling markers were measured at 0,6,12,15,18, and 24 and areal BMD of the distal radius, spine, and hip were measured at 0,12,18 and 24 months.
Results
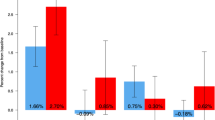
After denosumab discontinuation, serum markers of bone remodeling remained suppressed when followed by alendronate, but gradually increased to baseline when followed by raloxifene. In the denosumab-to-alendronate group, denosumab-induced BMD gains were maintained at all sites whereas in the denosumab-to-raloxifene group, BMD decreased at the spine by 2.0% (95% CI -3.2 to -0.8, P = 0.003) and at the total hip by 1.2% (-2.1 to -0.4%, P = 0.008), but remained stable at the femoral neck and distal radius and above the original baseline at all sites. The decreases in spine and total hip BMD in the denosumab-to-raloxifene group (but not the femoral neck or distal radius) were significant when compared to the denosumab-to-alendronate group.
Conclusions
These results suggest that after one year of denosumab, one year of alendronate is better able to maintain the inhibition of bone remodeling and BMD gains than raloxifene.




Similar content being viewed by others
Change history
09 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00198-023-06966-6
References
Bone HG, Wagman RB, Brandi ML et al (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5:513–523
Tsai JN, Uihlein AV, Burnett-Bowie SM, Neer RM, Derrico NP, Lee H, Bouxsein ML, Leder BZ (2016) Effects of Two Years of Teriparatide, Denosumab, or Both on Bone Microarchitecture and Strength (DATA-HRpQCT study). J Clin Endocrinol Metab 101:2023–2030
McClung MR, Lewiecki EM, Cohen SB et al (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354:821–831
Cummings SR, San Martin J, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Lacey DL, Timms E, Tan HL et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Yasuda H, Shima N, Nakagawa N et al (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95:3597–3602
Brown JP, Prince RL, Deal C et al (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Mineral Res 24:153–161
Reid IR, Miller PD, Brown JP et al (2010) Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res 25:2256–2265
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, San Martin J, Amg Bone Loss Study G (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43:222–229
McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM (2017) Observations following discontinuation of long-term denosumab therapy. Osteoporos Int 28:1723–1732
Zanchetta MB, Boailchuk J, Massari F, Silveira F, Bogado C, Zanchetta JR (2018) Significant bone loss after stopping long-term denosumab treatment: a post FREEDOM study. Osteoporos Int 29:41–47
Brown JP, Dempster DW, Ding B, Dent-Acosta R, San Martin J, Grauer A, Wagman RB, Zanchetta J (2011) Bone remodeling in postmenopausal women who discontinued denosumab treatment: off-treatment biopsy study. J Bone Miner Res 26:2737–2744
Seeman E ZR, Zanchetta JR, Hanley DA, Wang A, Libanti C, Wagman RB (2017) Bone microarchitecture after discontinuation of denosumab in postmenopausal women with low bone mass. J Bone Miner Res 31:Supplement 1
Brown JP, Roux C, Torring O et al (2013) Discontinuation of denosumab and associated fracture incidence: analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial. J Bone Miner Res 28:746–752
Cummings SR, Ferrari S, Eastell R et al (2018) Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. J Bone Miner Res 33:190–198
Cosman F, Huang S, McDermott M, Cummings SR (2022) Multiple Vertebral Fractures After Denosumab Discontinuation: FREEDOM and FREEDOM Extension Trials Additional Post Hoc Analyses. J Bone Miner Res 37:2112–2120
Anastasilakis AD, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Makras P (2019) Zoledronate for the Prevention of Bone Loss in Women Discontinuing Denosumab Treatment. A Prospective 2-Year Clinical Trial. J Bone Miner Res 34:2220–2228
Makras P, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Anastasilakis AD (2020) The three-year effect of a single zoledronate infusion on bone mineral density and bone turnover markers following denosumab discontinuation in women with postmenopausal osteoporosis. Bone 138:115478
Kendler D, Chines A, Clark P, Ebeling PR, McClung M, Rhee Y, Huang S, Stad RK (2020) Bone mineral density after transitioning from denosumab to alendronate. J Clin Endocrinol Metab 105(3):e255–e264
Ramchand SK, David NL, Lee H, Eastell R, Tsai JN, Leder BZ (2021) Efficacy of Zoledronic Acid in Maintaining Areal and Volumetric Bone Density After Combined Denosumab and Teriparatide Administration: DATA-HD Study Extension. J Bone Miner Res 36:921–930
Horne AM, Mihov B, Reid IR (2019) Effect of Zoledronate on Bone Loss After Romosozumab/Denosumab: 2-Year Follow-up. Calcif Tissue Int 105:107–108
Reid IR, Horne AM, Mihov B, Gamble GD (2017) Bone Loss After Denosumab: Only Partial Protection with Zoledronate. Calcif Tissue Int 101:371–374
Everts-Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann T (2020) A Single Infusion of Zoledronate in Postmenopausal Women Following Denosumab Discontinuation Results in Partial Conservation of Bone Mass Gains. J Bone Miner Res 35:1207–1215
Solling AS, Harslof T, Langdahl B (2021) Treatment With Zoledronate Subsequent to Denosumab in Osteoporosis: A 2-Year Randomized Study. J Bone Miner Res 36:1245–1254
Bonnick SL, Johnston CC Jr, Kleerekoper M, Lindsay R, Miller P, Sherwood L, Siris E (2001) Importance of precision in bone density measurements. J Clin Densitom 4:105–110
McDonald MM, Khoo WH, Ng PY et al (2021) Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 184:1940
Fu Q, Bustamante-Gomez NC, Reyes-Pardo H et al (2023) Reduced osteoprotegrin expression by osteocytes may contribute to rebound resorption after denosumab discontinuation. J Clin Invest Insight 8(18):e167790. https://doi.org/10.1172/jci.insight.167790
Jahn-Rickert K, Wolfel EM, Jobke B, Riedel C, Hellmich M, Werner M, McDonald MM, Busse B (2020) Elevated Bone Hardness Under Denosumab Treatment, With Persisting Lower Osteocyte Viability During Discontinuation. Front Endocrinol 11:250
Johnell O, Scheele WH, Lu Y, Reginster JY, Need AG, Seeman E (2002) Additive effects of raloxifene and alendronate on bone density and biochemical markers of bone remodeling in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 87:985–992
Ott SM, Oleksik A, Lu Y, Harper K, Lips P (2002) Bone histomorphometric and biochemical marker results of a 2-year placebo-controlled trial of raloxifene in postmenopausal women. J Bone Miner Res 17:341–348
Ha J, Kim J, Jeong C, Lim Y, Kim MK, Kwon HS, Song KH, Kang MI, Baek KH (2022) Effect of follow-up raloxifene therapy after denosumab discontinuation in postmenopausal women. Osteoporos Int 33:1591–1599
Hong N, Shin S, Lee S, Kim KJ, Rhee Y (2022) Raloxifene Use After Denosumab Discontinuation Partially Attenuates Bone Loss in the Lumbar Spine in Postmenopausal Osteoporosis. Calcif Tissue Int 111:47–55
Greenspan S, Field-Munves E, Tonino R, Smith M, Petruschke R, Wang L, Yates J, de Papp AE, Palmisano J (2002) Tolerability of once-weekly alendronate in patients with osteoporosis: a randomized, double-blind, placebo-controlled study. Mayo Clin Proc 77:1044–1052
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Chesnut CH 3rd, Skag A, Christiansen C et al (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Jha S, Wang Z, Laucis N, Bhattacharyya T (2015) Trends in Media Reports, Oral Bisphosphonate Prescriptions, and Hip Fractures 1996–2012: An Ecological Analysis. J Bone Miner Res 30:2179–2187
Black DM, Rosen CJ (2016) Postmenopausal Osteoporosis. N Engl J Med 374:2096–2097
Ettinger B, Black DM, Mitlak BH et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637–645
Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, Secrest RJ, Cummings SR, Investigators C (2004) Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 96:1751–1761
Gallant MA, Brown DM, Hammond M, Wallace JM, Du J, Deymier-Black AC, Almer JD, Stock SR, Allen MR, Burr DB (2014) Bone cell-independent benefits of raloxifene on the skeleton: a novel mechanism for improving bone material properties. Bone 61:191–200
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
JNT has a financial interest in Amgen. JNT’s interests were reviewed and are managed by MGH and Mass General Brigham in accordance with their conflict-of-interest policies. All other authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sabashini K. Ramchand and Joy N. Tsai are equal first authors.
The original online version of this article was revised: the ‘Co-first authorship’ note was missing, and should have read that Sabashini K. Ramchand and Joy N. Tsai are equal first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramchand, S.K., Tsai, J.N., Lee, H. et al. The comparison of alendronate and raloxifene after denosumab (CARD) study: A comparative efficacy trial. Osteoporos Int 35, 255–263 (2024). https://doi.org/10.1007/s00198-023-06932-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06932-2