Abstract
Aims/hypothesis
We postulated that the increased lifetime risk of chronic kidney disease (CKD) in young-onset diabetes is attributable to both long disease duration and more aggressive disease. We examined whether age at diabetes diagnosis modifies the effect of diabetes duration on risk of CKD.
Methods
We included 436,744 people with incident type 2 diabetes in the Hong Kong Diabetes Surveillance Database (HKDSD) and 16,979 people with prevalent type 2 diabetes in the Hong Kong Diabetes Register (HKDR). We used Poisson models to describe joint effects of age at diabetes diagnosis, diabetes duration and attained age on incidence of CKD in HKDSD. We used Cox proportional hazards models to examine interaction effect of age at diabetes diagnosis and diabetes duration on risk of CKD with adjustment for confounders in HKDR.
Results
During a median follow-up of 5.3 years, 134,043 cases of CKD were recorded in the HKDSD. The incidence rate ratio for CKD comparing people of the same attained age but diagnosed with diabetes at ages 5 years apart was higher for people with a younger age at diabetes diagnosis, but decreased with increasing age at diabetes diagnosis. During a median follow-up of 6.3 years, 6500 people developed CKD in the HKDR. The increased risk of CKD with longer diabetes duration decreased with older age at diabetes diagnosis. The adjusted HR for CKD associated with 5 year increase in diabetes duration was 1.37 (95% CI 1.13, 1.65) in people with diabetes diagnosed at 20–29 years and 1.01 (95% CI 0.87, 1.18) in those diagnosed at ≥70 years.
Conclusions/interpretation
Young age at diabetes diagnosis amplified the effect of increasing diabetes duration on increased risk of CKD.
Graphical abstract

Similar content being viewed by others

Introduction
The global epidemiology of diabetes is changing. Type 2 diabetes, once considered a disease of older adults, is increasingly being diagnosed in young people [1,2,3]. The number of young people, aged 20–39 years, living with type 2 diabetes had more than doubled from 23 million in 2000 to 63 million in 2013 worldwide, with the most rapid increases occurring in the Western Pacific region [4]. Chronic kidney disease (CKD) is one of the major complications of diabetes and develops in about 50% of people with diabetes throughout the life course [3]. People with CKD may progress to develop kidney failure requiring kidney replacement therapy. Chronic kidney disease is also associated with ten- and threefold increased hazards of all-cause mortality and atherosclerotic CVD, respectively [5].
People with younger age at diabetes diagnosis, compared with those at older age of diagnosis, are at an increased lifetime risk of microvascular complications [6,7,8,9,10,11,12]. However, it is unclear whether this is fully attributable to longer disease exposure or the inherently aggressive nature of young-onset diabetes. A meta-analysis reported that each 1 year increase in age at diabetes diagnosis was associated with a 5% decreased risk of microvascular disease after adjustment for current age, but with a 5% increased risk when adjusting for diabetes duration [12]. These findings indicate a complex interplay between age at diabetes diagnosis, diabetes duration and age attainment on the risk of complications. As age attainment is the sum of age at diabetes diagnosis and diabetes duration with the three variables being linearly correlated, disentangling true effect of age at diabetes diagnosis is challenging and requires different approaches. We hypothesised that younger age at diabetes onset increases the susceptibility to CKD beyond the effect of longer diabetes duration. To test this hypothesis, we examined whether age at diabetes diagnosis modified the effect of diabetes duration on the risk of CKD using data from both territory-wide population-based and hospital registry-based diabetes cohorts.
Methods
Data source and study population
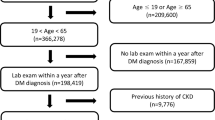
Details on the Hong Kong Diabetes Surveillance Database (HKDSD) [2, 13,14,15] and the Hong Kong Diabetes Register (HKDR) [16, 17] have been reported. Briefly, the Hong Kong Hospital Authority (HKHA) is a statutory body established in 1990 that governs all public hospitals and clinics and provides about 90% of total health services in Hong Kong [18]. In 2000, the HKHA adopted an electronic medical record system to capture clinical information on all people attending public hospitals and clinics. The HKDSD is a territory-wide cohort of people with diabetes identified from the HKHA electronic medical record system. Diabetes was ascertained using laboratory tests (HbA1c ≥ 48 mmol/mol [6.5%] or fasting plasma glucose ≥7.0 mmol/l), prescription of insulin or oral glucose-lowering drugs (OGLDs), and/or diagnosis codes for diabetes by physicians [2]. Types of diabetes were identified using a validated algorithm [19]. For this study, we included 436,744 people with incident type 2 diabetes in the HKDSD between 2002 and 2015 who were aged 20 years or older and had no CKD at the time of diabetes diagnosis. The participant selection for analysis in the HKDSD is shown in ESM Fig. 1.
The HKDR is a research-driven quality improvement programme established in 1995 at the Prince of Wales Hospital (PWH), the teaching hospital of the Chinese University of Hong Kong. The HKDR consecutively enrolled people with physician-diagnosed diabetes who were referred to the PWH Diabetes and Endocrine Centre for comprehensive assessment of metabolic control and diabetes complications. Referral sources included hospital-based specialty clinics, community clinics and private general practitioners. All people underwent structured evaluation at the time of enrolment, including demographic status, age at diabetes diagnosis, medical history, drug use, anthropometric measurements and laboratory tests. For this study, we included 16,979 people with type 2 diabetes enrolled in the HKDR between 1995 and 2015 who were aged 20 years or older at diabetes diagnosis and free of CKD at the time of enrolment. The participant selection for analysis in the HKDR is shown in ESM Fig. 2. In both HKDSD and HKDR, a larger proportion of people with pre-existing CKD at baseline were excluded in the group diagnosed with diabetes at an older than younger age (ESM Table 1). Compared with those without CKD at baseline, people with pre-existing CKD generally had a worse metabolic profile and a higher prevalence of comorbidities (ESM Table 2).
Outcome ascertainment
Follow-up data including serial measurements of serum creatinine and procedure codes for both HKDSD and HKDR were retrieved from the HKHA medical record system. Information on death was obtained by linkage to the Hong Kong Death Registry using the unique Hong Kong Identity Card number, which is compulsory for all residents in Hong Kong [13]. People with incident CKD were defined as having an eGFR <60 ml min−1 [1.73 m]−2 using the CKD Epidemiology Collaboration formula [20], and requiring dialysis (ICD-9 procedure code 39.95 or 54.98) or kidney transplant (ICD-9 procedure codes: 55.6). The date of incident CKD was the date of the first episode fulfilling the criteria of CKD.
Statistical analysis
Using population-based data of people with incident type 2 diabetes from the HKDSD, we calculated the incidence rates of CKD according to age at diabetes diagnosis, diabetes duration and age attainment (Fig. 1). People were followed up from date of diabetes diagnosis to date of first record of incident CKD, death or 31 December 2016, whichever came first. Follow-up time was divided into 6-month intervals by age attainment, diabetes duration and calendar year. Poisson models were used to predict CKD incidence rates for men and women separately. We fitted Poisson models using the log of follow-up time as the offset variable and natural spline effects of age at diabetes diagnosis, duration of diabetes and age attainment, with a linear effect of calendar time. Although the Poisson models were over-parametrised given the relationship between age at diabetes diagnosis, duration of diabetes and age attainment, they had no impact on the predictions obtained because the predictions did not depend on specific parametrisation [21]. We generated incidence rate ratios of CKD between people of the same attained age but with a 5 year separation in age at diabetes diagnosis (corresponding to a 5 year difference in diabetes duration) over a follow-up time of 10 years. For example, we computed the rate ratio of CKD for people with diabetes diagnosed at age 25 vs 30 years when both had reached the same age anywhere between 30 and 40 years (ESM Fig. 3). The rate ratios of CKD for the pair with diabetes diagnosis at age 25 vs 30 years were then plotted against attained age from 30 to 40 years (Fig. 2). These calculations were repeated for people diagnosed with diabetes at age 35 vs 40 years, 45 vs 50 years, and so on. The joint effects of age at diabetes diagnosis, diabetes duration and attained age on incidence of CKD were described by comparing the rate ratios of CKD between the different age pairs [21]. The same analyses were conducted for people diagnosed with diabetes at ages 10 years apart with a follow-up time of 5 years.
Incidence rates of CKD in people with incident type 2 diabetes according to age at diabetes diagnosis in the HKDSD. The curves represent the incidence rates of CKD in men (blue lines) and women (red lines) with type 2 diabetes diagnosed at the age of 20, 30, 40, 50, 60 and 70 years, respectively, indicated by the dashed grey vertical lines. Each curve shows the joint effects of age at diabetes diagnosis, diabetes duration and current age. The shaded areas represent the 95% CIs, estimated using the R package Epi. The y-axis is on a natural logarithmic scale
Incidence rate ratios for CKD associated with 5 and 10 year longer duration of diabetes in people with type 2 diabetes according to age at diabetes diagnosis in the HKDSD. The curves represent the incidence rate ratios for CKD associated with 5 year (a) and 10 year (b) longer duration of diabetes. The dashed grey vertical lines in (a) show the rate ratios for CKD comparing people with type 2 diabetes diagnosed at ages 25 vs 30, 35 vs 40, 45 vs 50, 55 vs 60, 60 vs 65, 65 vs 70 and 70 vs 75 years, respectively, when they have both reached the same age as indicated by the x-axis. The dashed grey vertical lines in (b) show the rate ratios for CKD comparing people with type 2 diabetes diagnosed at ages 20 vs 30, 30 vs 40, 40 vs 50, 50 vs 60, 55 vs 65, 60 vs 70 and 65 vs 75 years, respectively, when both have reached the same age as indicated by the x-axis. Each curve thus shows the joint effects of age at diabetes diagnosis, diabetes duration and current age. The blue lines represent men and red lines represent women. The shaded areas represent the 95% CIs, estimated using the R package Epi. The y-axis is on a natural logarithmic scale
Since the crude rate ratios might be affected by confounders such as smoking, BMI, BP and other comorbidities which were not available in the HKDSD, we verified these findings by examining the hospital registry-based data of people with prevalent type 2 diabetes from the HKDR who had undergone comprehensive assessment at enrolment. We examined whether the interaction effect of age at diabetes diagnosis and diabetes duration on the risk of CKD was independent of metabolic control, comorbidities, treatment and other potential confounders. We performed a complete case analysis excluding people with missing data at enrolment and compared the characteristics of people with complete data with those of the entire HKDR cohort. People were followed up from enrolment until the first record of incident CKD, death or 30 June 2017, whichever came first. We fitted Cox proportional hazards models to estimated HRs for incident CKD by diabetes duration at enrolment and adjusted for potential confounders including age at diabetes diagnosis; sex; smoking status; BMI; systolic BP; HbA1c; LDL-cholesterol; HDL-cholesterol; log (triacylglycerols); eGFR; log (urinary albumin/creatinine ratio [ACR]); history of CVD, cancer, diabetic retinopathy and sensory neuropathy; use of OGLDs, insulin, lipid-lowering drugs, BP-lowering drugs and renin-angiotensin system inhibitors; and enrolment year. We then compared the HRs across strata of age at diabetes diagnosis (20–29, 30–39, 40–49, 50–59, 60–69 and 70 years or above). We applied a restricted cubic spline analysis with five knots at 10%, 25%, 50%, 75% and 90% through the total distribution of diabetes duration and found that the shape of the association between diabetes duration and risk of CKD was linear (p for non-linearity =0.96) (ESM Fig. 4). Thus, we treated diabetes duration as a continuous variable in Cox proportional hazards models and reported HRs for incident CKD associated with each 5 year increase in diabetes duration. There was no evidence of violation of the proportional hazard assumption checked using Schoenfeld residuals. To assess the effect of competing risk from death on CKD risk, we further fitted Fine–Gray models [22] to estimate sub-distribution hazard ratios (SHRs) with death as the competing event. To assess the effect of missing data, we used multiple imputation assuming data were missing at random. The imputation model included all covariates at enrolment as well as outcome event (incident CKD) and follow-up time. Five imputed datasets were generated to estimate the pooled HRs [23]. We also performed a sensitivity analysis excluding people who developed CKD within 1 year after diabetes diagnosis to minimise the potential impact of reverse causality. In addition, we compared the HRs for incident CKD associated with each 5 year increase in diabetes duration across strata of age attainment at enrolment. A two-sided significance level of 0.05 was considered statistically significant. All analyses were conducted with R software, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). This study was approved by the local Clinical Research Ethics Committee.
Results
The HKDSD (population-based cohort)
Among 436,744 people with newly diagnosed type 2 diabetes, 51.9% were men and the mean age at diabetes diagnosis was 59.7 (SD = 11.8) years (Table 1). During a median of 5.3 years (total 2,573,633 person-years) of follow-up, 134,043 cases of CKD (52.8% men) were recorded with an incidence rate of 52.1 per 1000 person-years. The incidence rates of CKD declined during the first year after diabetes diagnosis and then increased linearly by diabetes duration in both sexes and across all age groups of diabetes diagnosis (Fig. 1). Men generally had higher incidence rates of CKD than women at any given age (Fig. 1 and Table 1). However, the absolute difference in CKD rates between sexes diminished with increasing age, particularly in old people aged 70 years or above.
Figure 2 shows the joint effects of age at diabetes diagnosis, a 5 or 10 year difference in duration of diabetes and attained age on incidence of CKD by sex. The rate ratios for CKD comparing people of the same attained age but with a 5 year difference in duration of diabetes (longer vs shorter) started below 1.0 and ranged between 1.0 and 1.5 after the first half year of diagnosis (Fig. 2a). The rate ratios for CKD associated with 5 year longer diabetes duration diminished with older age at diabetes diagnosis. For example, men at the attained age of 34 years and diagnosed with diabetes at age of 25 years had 25% (rate ratio 1.25, 95% CI 1.20, 1.29) higher risk of CKD compared with men at the same age attainment (current age 34 years) but diagnosed with diabetes at age of 30 years (corresponding to a 5 year difference in diabetes duration). By comparison, the rate ratio for CKD was 1.05 (95% CI 1.02, 1.09) between men aged 79 years and diagnosed with diabetes at ages of 70 vs 75 years. A similar pattern as in decreasing rate ratio with increasing age at diabetes diagnosis was observed for a 10 year difference in diabetes duration on the risk of CKD (Fig. 2b). The effect of both a 5 year and a 10 year difference in duration of diabetes on the risk of CKD was greater among women than men (Fig. 2).
The HKDR (registry-based cohort)
Of the 16,979 people with complete data included in the analysis, 52.1% were men (Table 1). The mean ages at diabetes diagnosis and at enrolment were 51.0 (SD = 11.0) and 57.2 (SD = 10.9) years, respectively. People diagnosed with diabetes at younger age were younger, had longer diabetes duration, poorer glycaemic control, higher eGFR and BMI, lower systolic BP and urinary ACR, and were less likely to have comorbidities but were more likely to use insulin at enrolment (ESM Table 3). Distribution of characteristics of people with complete data and those of the entire cohort including people with missing data was similar (ESM Table 4).
During a median of 6.3 years (total 124,079 person-years) of follow-up, 6500 people (51.4% men) developed CKD with an incidence rate of 52.4 per 1000 person-years (Table 1). Among people with incident CKD, 65.0% had albuminuria, with the highest proportion in people with the youngest age at diabetes diagnosis and decreasing proportions with increasing age at diabetes diagnosis (ESM Table 5). After adjusting for potential confounders, each 5 year increase in diabetes duration was associated with 23% (95% CI 20%, 26%) increased risk of CKD, with the association significantly greater among women (HR 1.32, 95% CI 1.28, 1.37) than men (HR 1.13, 95% CI 1.09, 1.17) (p for interaction <0.001) (Fig. 3 and ESM Table 6). After stratification for age at diabetes diagnosis, the effect of each 5 year increase in diabetes duration on risk of CKD was greatest in the group diagnosed with diabetes at 20–29 years (HR 1.37, 95% CI 1.13, 1.65) and decreased with increasing age at diabetes diagnosis (p for linear trend =0.003) (Fig. 3 and ESM Table 6). In people diagnosed with diabetes at 70 years or above, there was no significant association between diabetes duration and risk of CKD (HR 1.01, 95% CI 0.87, 1.18). The Fine–Gray models yielded similar findings to the Cox proportional hazards models after accounting for competing risk from death (ESM Fig. 5). The results were unchanged in sensitivity analyses using multiple imputation for missing data (ESM Fig. 6) or excluding people (n = 42) who developed CKD within 1 year after diabetes diagnosis (data are not shown). The effect of each 5 year increase in diabetes duration on risk of CKD did not decrease linearly with increasing age attainment at enrolment (p for linear trend =0.47) (ESM Fig. 7).
HRs for incident CKD associated with 5 year increase in diabetes duration in people with prevalent type 2 diabetes stratified by age at diabetes diagnosis in the HKDR. The Cox models included duration of diabetes, age at diabetes diagnosis, sex, BMI, HbA1c, LDL-cholesterol, HDL-cholesterol, log (triacylglycerol), systolic BP, eGFR, log (urinary ACR), smoking status, history of CVD, cancer, diabetic retinopathy and sensory neuropathy, use of OGLDs, insulin, lipid-lowering drugs, BP-lowering drugs and renin–angiotensin system inhibitors and enrolment year. The area of the square is proportional to the size of the study population. The horizontal lines represent the 95% CIs of HRs. The horizontal axis for HRs is on a natural logarithmic scale. All p values for interaction between age at diabetes diagnosis and diabetes duration on risk of CKD in the groups of both sexes and men and women separately were <0.05
Discussion
Using data from both territory-wide population-based cohort of people with incident diabetes and hospital registry-based cohort of people with prevalent diabetes, we report two key findings. First, our study provides evidence that younger age at diabetes diagnosis affected the risk of CKD beyond the effect of longer disease duration. Young age at diabetes diagnosis amplified the effect of increasing diabetes duration on increased risk of CKD, even after adjustment for metabolic risk profile and other confounders. Second, although men had a higher absolute incidence of CKD than women, the adverse effect of increasing duration of diabetes on risk of CKD was greater for women than men.
Comparison with other studies
A number of studies have sought to investigate the direct association between age at diabetes diagnosis and risk of complications including death [12]. Some data showed that age at diabetes diagnosis was inversely associated with microvascular complications in the univariable analysis, but was rendered non-significant upon further adjustment for diabetes duration [7]. In a prospective analysis of over 1 million people with type 2 diabetes in Australia, the incidence rates of treated kidney failure were higher in people with older age at diabetes diagnosis in the first two decades of disease and reversed thereafter with increasing diabetes duration [24]. The findings from these studies suggested that the higher cumulative risks of kidney complications in people with younger age at diabetes onset were mainly driven by longer disease duration. In most of these studies, the independent influence of younger age at development of diabetes and duration of diabetes on the rate of progression of complications have not been separately examined and it remained unclear whether younger age at diabetes diagnosis might interact with increasing diabetes duration to increase the risk of kidney complications.
Diabetes duration is a known determinant for CKD and reflects the cumulative impact of high blood glucose and other related aberrations on the kidney. In an analysis of data from the ADVANCE RCT including 11,140 people with type 2 diabetes, age at diabetes diagnosis was not associated with the risk of microvascular complications after adjustment for baseline HbA1c levels [6]. An interaction was detected between age at diabetes diagnosis and diabetes duration on microvascular events, but detailed analysis was not reported. In the present study, we compared the effect of diabetes duration on the risk of CKD according to strata of age at diabetes diagnosis. We adopted the approach used in the study by Huo and colleagues [21]. We found that the increased risk of CKD conferred by longer disease duration amplified with younger age at diabetes diagnosis, thus supporting our hypothesis that young-onset diabetes heightens the propensity for kidney disease beyond the effect of longer disease duration.
Higher risk of CKD among people with young-onset diabetes
The mechanisms linking younger age at diabetes diagnosis with higher risk for CKD are multifactorial. First, people with young-onset diabetes have worse glycaemic control and higher BMI compared with their late-onset counterparts [25, 26]. Other researchers have reported that beta cell function declines at a faster rate in young people [27], who are also more insulin resistant because of higher burden of obesity [28]. Moreover, the responses to OGLDs differ by age at diabetes diagnosis. Compared with late-onset diabetes, those with young-onset diabetes have smaller responses to most combinations of OGLDs, resulting in more rapid deterioration in HbA1c and up to a threefold higher glycaemic burden over their lifetime [29]. Second, people with young-onset diabetes are more likely to have an unhealthy lifestyle (e.g. smoking) and are less likely to receive medication treatments and achieve targets for lipids [25], which are also risk factors for CKD. This could be due to a combination of delay in seeking medical care, poor medication adherence, clinical inertia and lack of treatment guideline. However, the modifying effect of age at diabetes diagnosis on disease duration remained significant when we adjusted for these risk factors, suggesting that there are other contributory factors. Third, kidney tissues might be more vulnerable to insults from high glucose milieu when exposed at a younger age. More pronounced inflammatory and fibrotic responses to glucose have been reported in young individuals with diabetes [30]. Lastly, genetic variants that predisposed to early diabetes development might increase the susceptibility to kidney complications through non-glucose mediating pathways [31, 32].
In contrast, among people diagnosed at 70 years of age or older, longer duration of diabetes did not increase the risk of CKD. In our previous analysis, we noted stable glycaemic control in people diagnosed with diabetes aged 60 years and glycaemic improvements in those diagnosed at older ages [29]. In these older survivors, age-related factors and comorbid conditions were likely to be more important factors than disease duration in the development of CKD. Moreover, the proportion of people excluded from Cox regression analysis because of pre-existing CKD was 40% in the group diagnosed with diabetes at age of 70 years or above vs 8% in the youngest age group in the HKDR. This has resulted in a larger proportion of healthier population being included in analysis in the oldest compared with the youngest age group at diabetes diagnosis. Although this is unlikely to invalidate our findings as we focused on incident CKD and the prevalence of CKD is high among old people regardless of diabetes status [3, 33], it would be worthwhile to explore whether the association between diabetes duration and risk of CKD reported in our study applies to a relatively sick and older population. In the HKDR, the diminishing effect of longer diabetes duration on CKD with increasing age at diabetes diagnosis seemed to be mainly driven by the results in the youngest and the oldest age group, which had the smallest sample size. Although the absolute differences in HRs across the middle-age groups were small, a linear decrease in the point estimate of the effect with increasing age at diabetes diagnosis was observed. Studies with a larger population in the youngest and the oldest age groups are warranted to confirm our findings.
We observed a surge in the incidence of CKD around the time of diabetes diagnosis, which declined during the first half year and increased in subsequent years in both men and women (Fig. 1). We speculated that this early rise might be due to detection of undiagnosed cases of CKD upon diagnosis of diabetes when laboratory tests were performed. We noted that people with younger age at diabetes diagnosis had a higher initial eGFR at enrolment in the HKDR. Thus, upon reaching CKD, the extent of eGFR decline would have been greater in the younger than the older group. This suggests that the actual adverse effect of younger age at diabetes diagnosis on increased risk of CKD might be greater than that reported in our study. Consistent with other studies showing that albuminuria is the most common initial manifestation of diabetic kidney disease especially in young people, we also found a higher proportion of albuminuric CKD in those diagnosed with diabetes at a younger age [34].
Ahlqvist et al used data from Caucasians and revealed that among the five clusters of diabetes, the cluster characterised by severe insulin resistance and high BMI was associated with the highest risk of CKD [35]. Obesity is common in young people with diabetic kidney disease [36]. In the HKDR, participants with younger age at diabetes diagnosis had higher BMI than their older counterparts although the difference was only modest. It is noteworthy that the relationship between BMI and visceral adiposity varies across ethnic groups; East Asians have more visceral fat than Caucasians with the same BMI [37]. Hence, BMI may not be as effective in reflecting insulin resistance in our Chinese population, and this may diminish the association between BMI and CKD.
Sex difference in the effect of diabetes duration on risk of CKD
Male sex has been reported as an independent risk factor for CKD [38,39,40,41,42]. In our study, the absolute incidence rates of CKD were greater in men than women across almost the full age spectrum. Diabetic kidney disease rarely developed during childhood but might progress during puberty with a surge of testosterone [31]. The latter might upregulate the renin-angiotensin system to promote sodium reabsorption and increase intraglomerular pressure in the kidney to drive progression of kidney diseases in men [43]. The high levels of oestrogen in women protect against the development of kidney disease by attenuating the effect of profibrotic and proinflammatory factors such as high blood glucose [42]. The decline of oestrogen after menopause in women, accompanied by an increase in risk of CKD, might have contributed to the reduction in sex differences in CKD rates with increasing age. Compared with men, women were less likely to have risk factors such as smoking and the metabolic syndrome, making diabetes duration a more prominent risk factor for kidney disease [38].
Strengths and limitations
The major strengths of this study include the large sample size and long follow-up period. We analysed two complementary cohorts with incident and prevalent diabetes who had a wide range of diabetes duration which yielded consistent results. Our study also has limitations. First, around 10% of people treated in the private sector are not captured in the HKDSD [18]. Characteristics may differ between people receiving care in the public and private sector. In addition, the HKDR is a hospital registry-based cohort and is not fully representative of the total population with diabetes in Hong Kong. People in the HKDR were generally sicker than those in the HKDSD. However, low representativeness does not necessarily hamper scientific inference [44,45,46]. Controlling for confounding, the large number of participants with different diabetes duration, and long-term follow-up are expected to provide valid inferences. Second, similar to most epidemiological studies, CKD was ascertained based on a single eGFR measurement with potential misclassification bias. Transient decline in eGFR due to acute kidney injury or concurrent illnesses remained a possibility. However, transient drops in eGFR due to acute kidney injury were rare compared with drops due to CKD and were unlikely to seriously impact the results. Third, we could not exclude CKD due to causes other than diabetes. Nonetheless, people with CKD often have mixed aetiologies including hypertension and obesity, and it is difficult to dissect the contribution of each cause clinically. Fourth, in the HKDSD, lifestyle and sociodemographic information was not available and we could only describe the unadjusted effect of age at diabetes diagnosis and diabetes duration on incident CKD. Although most metabolic risk factors were available in the HKDR, residual/unmeasured confounding (e.g. socioeconomic status and psychosocial stress) remained possible [47]. Fifth, further sub-analysis by stratifying CKD stages was not feasible because of the very small event number in young people. Finally, these findings from a predominantly Chinese population might not generalise to other populations, although the high risk for CKD in people with young-onset diabetes is now well recognised [3].
In conclusion, our data suggest that the higher lifetime risk of CKD among people with younger age at diabetes diagnosis is not simply driven by prolonged disease duration. Young age at diabetes diagnosis amplified the effect of increasing diabetes duration on increased risk of CKD. In view of the rising prevalence of young-onset diabetes, our findings have important public and personal health implications. Intensive control of risk factors, notably obesity and BP, and timely use of organ-protective drugs in young people with diabetes is needed to curb this rising burden of kidney disease. More research is needed to understand the pathophysiology of CKD in the context of early age of diabetes onset.
Data availability
The data that support the findings of this study are available from the corresponding author (AOYL) upon reasonable request.
Abbreviations
- ACR:
-
Albumin/creatinine ratio
- CKD:
-
Chronic kidney disease
- HKDR:
-
Hong Kong Diabetes Register
- HKDSD:
-
Hong Kong Diabetes Surveillance Database
- HKHA:
-
Hong Kong Hospital Authority
- OGLDs:
-
Oral glucose-lowering drugs
- PWH:
-
Prince of Wales Hospital
- SHR:
-
Sub-distribution HR
References
Geiss LS, Wang J, Cheng YJ et al (2014) Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 312(12):1218–1226. https://doi.org/10.1001/jama.2014.11494
Luk AOY, Ke C, Lau ESH et al (2020) Secular trends in incidence of type 1 and type 2 diabetes in Hong Kong: a retrospective cohort study. PLoS Med 17(2):e1003052. https://doi.org/10.1371/journal.pmed.1003052
Thomas MC, Cooper ME, Zimmet P (2016) Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol 12(2):73–81. https://doi.org/10.1038/nrneph.2015.173
Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S (2018) Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol 6(1):69–80. https://doi.org/10.1016/s2213-8587(17)30186-9
So WY, Kong AP, Ma RC et al (2006) Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care 29(9):2046–2052. https://doi.org/10.2337/dc06-0248
Zoungas S, Woodward M, Li Q et al (2014) Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 57(12):2465–2474. https://doi.org/10.1007/s00125-014-3369-7
Chan JCN, Lau ESH, Luk AOY et al (2014) Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am J Med 127(7):616–624. https://doi.org/10.1016/j.amjmed.2014.03.018
Chuang LM, Soegondo S, Soewondo P et al (2006) Comparisons of the outcomes on control, type of management and complications status in early onset and late onset type 2 diabetes in Asia. Diabetes Res Clin Pract 71(2):146–155. https://doi.org/10.1016/j.diabres.2005.05.007
Wong J, Molyneaux L, Constantino M, Twigg SM, Yue DK (2008) Timing is everything: age of onset influences long-term retinopathy risk in type 2 diabetes, independent of traditional risk factors. Diabetes Care 31(10):1985–1990. https://doi.org/10.2337/dc08-0580
Nanayakkara N, Ranasinha S, Gadowski A et al (2018) Age, age at diagnosis and diabetes duration are all associated with vascular complications in type 2 diabetes. J Diabetes Complicat 32(3):279–290. https://doi.org/10.1016/j.jdiacomp.2017.11.009
Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE (2020) Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol 16(6):321–331. https://doi.org/10.1038/s41574-020-0334-z
Nanayakkara N, Curtis AJ, Heritier S et al (2020) Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses. Diabetologia https://doi.org/10.1007/s00125-020-05319-w
Ke C, Lau E, Shah BR et al (2019) Excess burden of mental illness and hospitalization in young-onset type 2 diabetes: a population-based cohort study. Ann Intern Med 170(3):145–154. https://doi.org/10.7326/M18-1900
Wu H, Lau ESH, Ma RCW et al (2020) Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001–2016: a retrospective cohort study. Diabetologia 63(4):757–766. https://doi.org/10.1007/s00125-019-05074-7
Wu H, Lau ESH, Yang A et al (2020) Trends in diabetes-related complications in Hong Kong, 2001–2016: a retrospective cohort study. Cardiovasc Diabetol 19(1):60. https://doi.org/10.1186/s12933-020-01039-y
Chan JCN, Lim L-L, Luk AOY et al (2019) From Hong Kong Diabetes Register to JADE Program to RAMP-DM for data-driven actions. Diabetes Care 42(11):2022–2031. https://doi.org/10.2337/dci19-0003
Luk AOY, Lau ESH, Lim C et al (2019) Diabetes-related complications and mortality in patients with young-onset latent autoimmune diabetes: a 14-year analysis of the prospective Hong Kong Diabetes Register. Diabetes Care 42(6):1042–1050. https://doi.org/10.2337/dc18-1796
Census and Statistics Department (2013) Thematic Household Survey Report No. 50. Available from https://www.statistics.gov.hk/pub/B11302502013XXXXB0100.pdf. Accessed 16 Feb 2021
Ke C, Stukel TA, Luk A et al (2020) Development and validation of algorithms to classify type 1 and 2 diabetes according to age at diagnosis using electronic health records. BMC Med Res Methodol 20(1):1–15. https://doi.org/10.1186/s12874-020-00921-3
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Huo L, Magliano DJ, Rancière F et al (2018) Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997–2011. Diabetologia 61(5):1055–1063. https://doi.org/10.1007/s00125-018-4544-z
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94(446):496–509. https://doi.org/10.1080/01621459.1999.10474144
Marshall A, Altman DG, Holder RL, Royston P (2009) Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 9:57. https://doi.org/10.1186/1471-2288-9-57
Morton JI, Liew D, McDonald SP, Shaw JE, Magliano DJ (2020) The association between age of onset of type 2 diabetes and the long-term risk of end-stage kidney disease: a national registry study. Diabetes Care 43(8):1788–1795. https://doi.org/10.2337/dc20-0352
Yeung RO, Zhang Y, Luk A et al (2014) Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol 2(12):935–943. https://doi.org/10.1016/S2213-8587(14)70137-8
Wright AK, Welsh P, Gill JMR et al (2020) Age-, sex- and ethnicity-related differences in body weight, blood pressure, HbA1c and lipid levels at the diagnosis of type 2 diabetes relative to people without diabetes. Diabetologia 63(8):1542–1553. https://doi.org/10.1007/s00125-020-05169-6
RISE Consortium, RISE Consortium Investigators (2019) Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes 68(8):1670–1680. https://doi.org/10.2337/db19-0299
RISE Consortium (2018) Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. observations using the hyperglycemic clamp. Diabetes Care 41(8):1696–1706. https://doi.org/10.2337/dc18-0244
Ke C, Stukel TA, Shah BR et al (2020) Age at diagnosis, glycemic trajectories, and responses to oral glucose-lowering drugs in type 2 diabetes in Hong Kong: a population-based observational study. PLoS Med 17(9):e1003316–e1003316. https://doi.org/10.1371/journal.pmed.1003316
TODAY Study Group (2013) Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 36(6):1758–1764. https://doi.org/10.2337/dc12-2388
Franceschini N, Shara NM, Wang H et al (2012) The association of genetic variants of type 2 diabetes with kidney function. Kidney Int 82(2):220–225. https://doi.org/10.1038/ki.2012.107
Buraczynska M, Swatowski A, Markowska-Gosik D, Kuczmaszewska A, Ksiazek A (2011) Transcription factor 7-like 2 (TCF7L2) gene polymorphism and complication/comorbidity profile in type 2 diabetes patients. Diabetes Res Clin Pract 93(3):390–395. https://doi.org/10.1016/j.diabres.2011.05.017
GBD Chronic Kidney Disease Collaboration (2020) Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733. https://doi.org/10.1016/s0140-6736(20)30045-3
Maahs DM, Snively BM, Bell RA et al (2007) Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care 30(10):2593–2598. https://doi.org/10.2337/dc07-0450
Ahlqvist E, Storm P, Käräjämäki A et al (2018) Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 6(5):361–369. https://doi.org/10.1016/s2213-8587(18)30051-2
Maric-Bilkan C (2013) Obesity and diabetic kidney disease. Med Clin North Am 97(1):59–74. https://doi.org/10.1016/j.mcna.2012.10.010
Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN Jr (1994) Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr 60(1):23–28. https://doi.org/10.1093/ajcn/60.1.23
Luk AO, So WY, Ma RC et al (2008) Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: a 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care 31(12):2357–2361. https://doi.org/10.2337/dc08-0971
Nagata M, Ninomiya T, Doi Y et al (2010) Trends in the prevalence of chronic kidney disease and its risk factors in a general Japanese population: the Hisayama Study. Nephrol Dial Transplant 25(8):2557–2564. https://doi.org/10.1093/ndt/gfq062
Iseki K (2008) Gender differences in chronic kidney disease. Kidney Int 74(4):415–417. https://doi.org/10.1038/ki.2008.261
Iseki K, Iseki C, Ikemiya Y, Fukiyama K (1996) Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int 49(3):800–805. https://doi.org/10.1038/ki.1996.111
Clotet S, Riera M, Pascual J, Soler MJ (2016) RAS and sex differences in diabetic nephropathy. Am J Physiol Renal Physiol 310(10):F945–F957. https://doi.org/10.1152/ajprenal.00292.2015
Song J, Kost CK Jr, Martin DS (2006) Androgens potentiate renal vascular responses to angiotensin II via amplification of the Rho kinase signaling pathway. Cardiovasc Res 72(3):456–463. https://doi.org/10.1016/j.cardiores.2006.09.007
Rothman KJ, Gallacher JEJ, Hatch EE (2013) Why representativeness should be avoided. Int J Epidemiol 42(4):1012–1014. https://doi.org/10.1093/ije/dys223
Nohr EA, Olsen J (2013) Commentary: epidemiologists have debated representativeness for more than 40 years--has the time come to move on? Int J Epidemiol 42(4):1016–1017. https://doi.org/10.1093/ije/dyt102
Richiardi L, Pizzi C, Pearce N (2013) Commentary: representativeness is usually not necessary and often should be avoided. Int J Epidemiol 42(4):1018–1022. https://doi.org/10.1093/ije/dyt103
Wu H, Bragg F, Yang L et al (2019) Sex differences in the association between socioeconomic status and diabetes prevalence and incidence in China: cross-sectional and prospective studies of 0.5 million adults. Diabetologia 62(8):1420–1429. https://doi.org/10.1007/s00125-019-4896-z
Acknowledgements
We acknowledge the Hong Kong Hospital Authority for providing access to the electronic medical record.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HW contributed to conception of the article, statistical analysis, interpretation of results, drafting the manuscript, revising the manuscript critically for intellectual content and approving the final version to be published. AOYL and JCNC contributed to conception and design of the article, acquisition of data, interpretation of results, revising the manuscript critically and approving the final version to be published. AY, ESHL, BF, RCWM, APSK, EC and W-YS contributed to conception and design of the article, interpretation of results, revised the manuscript critically for intellectual content and approved the final version to be published. AOYL is the guarantor of this work, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM
(PDF 593 kb)
Rights and permissions
About this article
Cite this article
Wu, H., Lau, E.S.H., Yang, A. et al. Young age at diabetes diagnosis amplifies the effect of diabetes duration on risk of chronic kidney disease: a prospective cohort study. Diabetologia 64, 1990–2000 (2021). https://doi.org/10.1007/s00125-021-05494-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05494-4